Abstract
The present study is an exploration of a novel strategy to target a therapeutic gene to brain tumour tissues. In the present study, we evaluated the feasibility of using hMSCs (human mesenchymal stem cells) to deliver PEDF (pigment epithelium-derived factor), a potent inhibitor of tumour angiogenesis, in a model of intracranial gliomas. To assess its potential of tracking gliomas, MSCs (mesenchymal stem cells) were injected into the cerebral hemisphere and it showed that MSCs infiltrated into the vessel beds and scattered throughout the tumour. In vitro migration assay showed that the VEGF (vascular endothelial growth factor) enhanced MSC migration. In contrast, the migratory activity of MSCs was significantly inhibited with the presence of PEDF. Systematic delivery of AAV (adeno-associated virus)–PEDF to established glioma xenografts resulted in increased apoptosis of gliomas. In addition, MSC–PEDF treatment prolonged the survival of mice bearing U87 gliomas. Taken together, these data validate that MSCs–PEDF can migrate and deliver PEDF to target glioma cells, which may be a novel and promising therapeutic approach for refractory brain tumour.
Keywords: angiogenesis, glioma, mesenchymal stem cell, pigment epithelium-derived factor
Abbreviations: AAV, adeno-associated virus; DAB, diaminobenzidine; DMEM, Dulbecco’s modified Eagle’s medium; EGFP, enhanced green fluorescent protein; FBS, fetal bovine serum; hMSC, human mesenchymal stem cell; MSC, mesenchymal stem cell; MVD, microvessel density; PEDF, pigment epithelium-derived factor; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling; VEGF, vascular endothelial growth factor
INTRODUCTION
Angiogenesis is critical to sustaining the growth of tumour once it reaches a diameter of 1–2 mm [1]. Solid tumours depend on angiogenesis for growth and metastasis in a hostile environment [2]. Several anti-angiogenic drugs have therefore been developed to target tumour neovascularization. Glioma is a highly malignant brain tumour, which displays extensive neovascularization. Although many advances have recently been made in the conventional treatment of gliomas by surgery, radiotherapy combined with chemotherapy, this disease still has a very poor prognosis [3,4]. One major problem in the treatment of gliomas is their extreme invasiveness, which makes complete surgical removal unlikely and the gliomas relatively resistant to radiation and chemotherapy. Anti-angiogenic trails have been explored in malignant gliomas. Systemic administration of angiogenic inhibitors by viral-vector-mediated gene delivery and encapsulated producer cells has been investigated. Although these approaches of delivery could be promising, there are serious disadvantages, including short half-life of the systemically administered inhibitors, host immune response to viral vectors and inefficient intra-tumoral spread of viral vectors and encapsulated cells [5].
MSCs (mesenchymal stem cells), which reside within the stromal compartment of the bone marrow, have the capacity to differentiate into cells of connective tissue lineages and cells that are not a part of their normal repertoire [6,7]. Recently, it has been found that MSCs have the capacity of migrating towards gliomas after intracranial implantation or systemic delivery, which has made these cells an attractive therapeutic tool in gene therapy for gliomas [8,9].
PEDF (pigment epithelial-derived factor) is a 50-kDa secreted glycoprotein, which was initially identified as a neurotrophic factor. It was later discovered that PEDF has potent anti-angiogenic activity, far greater than any other known endogenously produced inhibitors of angiogenesis, including angiostatin, endostatin and thrombospondin-1 [10]. It has been reported that it activates the Fas/FasL death pathway and subsequently induces endothelial cell death, and also regulates the balance between pro-angiogenic and anti-angiogenic factors [11]. Previously, we demonstrated that PEDF plays an important role in angiogenesis and tumorigenesis of gliomas [12]. The aim of the present study was to investigate the tumour-targeting capacity of PEDF gene-modified MSCs in the glioma model.
MATERIALS AND METHODS
Cell lines and animals
The human malignant glioma cell line U87 and human fibroblasts were obtained from the Shanghai Institute of Cell Biology of the Chinese Academy of Sciences. The cells were cultured in the DMEM (Dulbecco's modified Eagle's medium) containing 10% FBS (fetal bovine serum), 100 units/ml penicillin and 100 μg/ml streptomycin at 37°C in humidified atmosphere containing 5% CO2. Male BALB/c-nu/nu mice (4–6 weeks old; Slac Laboratory Animal) were kept in the animal facilities at Fudan University and maintained under specific pathogen-free conditions. All animal procedures were conducted according to the guidelines approved by the China Association of Laboratory Animal Care.
Culture and identification of MSCs
MSCs were isolated and cultured as we routinely used and have previously described [13,14].
Phenotypical analysis of hMSCs (human mesenchymal stem cells) (a gift from the Department of Neurosurgery, Huashan Hospital, Fudan University, Shanghai, People's Republic of China) was performed by flow cytometry using FACSCalibur (Becton Dickinson). Briefly, the cells were washed twice with DPBS (Dulbecco's PBS) containing 0.1% BSA and then were labelled with phycoerythrin-labelled anti-human CD44, CD105, CD166, CD34, CD45 or CD11b/c monoclonal antibodies (Pharmingen). As an isotype-matched control, human IgG1- (R&D Systems) labelled cells were analysed. Samples were then fixed with 1% PFA (paraformaldehyde) before analysis with a FACStar II cell sorter (Becton Dickinson). Flow cytometric gates for MSCs were established based on forward and side scatter.
Infection of hMSCs with AAV (adeno-associated virus)–PEDF
The full-length cDNA of PEDF was cloned by RT–PCR (reverse transcriptase–PCR) as described previously [12]. The PCR products were then inserted between the NcoI and PacI sites of the AAV-2 expression vector to construct the AAV–PEDF. The integrity of the cDNA constructs was confirmed by DNA sequence analysis. AAV particles were generated using a three-plasmid and helper virus-free packaging system. AAV-2 vector encoding EGFP (enhanced green fluorescent protein) and LacZ (AAV–LacZ) were a generously provided by Dr. J.H. Zhu (Department of Neurosurgery, Huashan Hospital, Fudan University, Shanghai, People's Republic of China). MSCs or U87 cells were plated in a 6-cm plastic dish. After 24 h of cultivation, the cells were transfected with either AAV–PEDF or AAV–EGFP supernatant at an input multiplicity of infection of 500 in DMEM supplemented with 2% FBS, with normal saline as the non-infection control. The next day, the medium was replaced with the fresh medium containing 10% FBS. Supernatants were collected after culture for 6, 12, 24, 48, 72 h and stored at −80°C for further analysis.
Western blot assay
The supernatant was concentrated by super filter and mixed with an equal volume of SDS sample buffer. The identical amounts (25 μg) of protein were electro-blotted on to nitrocellulose membranes, and membranes were incubated with mouse anti-(human PEDF) monoclonal antibody (R&D Systems). After three washes with Tris-buffered saline with Tween, the membranes were incubated with HRP (horseradish peroxidase)-conjugated anti-mouse IgG (1:5000, Sigma). Immunoreactive bands were detected by an ECL® (enhanced chemiluminescence) Western blot analysis system (GE Healthcare).
Apoptosis analysis
Immunohistochemistry was used to detect apoptosis in MSC–PEDF-treated gliomas. MSC or MSC–PEDF was intravenously injected in mice with U87MG gliomas. After 15 days, the treated brains were harvested and stained for TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling). Apoptosis of tumours was assessed by in situ TUNEL analysis. Briefly, slides were incubated in 3% H2O2 for 10 min at room temperature (25°C) and then with 0.02% protease/PBS for 30 min at 37°C. After incubation with reaction buffer containing terminal deoxyribonucleotidyl transferase according to the manufacturer's protocol (Roche Molecular Biochemicals), the slides were stained with ABC reagent. Then, the slides were incubated with 3,3-DAB (diaminobenzidine)/H2O2 solutions and restained with 4% Methyl Green after complete washing.
In vitro cell migration assay
The cell migration assay was performed using double-chamber culture dishes (Transwell; Costar). MSCs labelled with EGFP (105) were placed in the upper chamber with 8 mm pores, and U87 cells, 50 ng/ml VEGF (vascular endothelial growth factor; Chemicon) and 50 ng/ml PEDF (Chemicon) were placed in the lower chamber. The Transwell was placed at 37°C, 5% CO2 for 48 h, and then the upper side of the filter was washed and scraped with a rubber policeman. Results of cell migration assays are expressed by counting the green MSCs in four random hpf (high-power fields) in each well using a fluorescence microscope (TE-2000, Nikon). Experiments were repeated three times separately.
Intracranial xenografting of human glioma cells
Mice were anaesthetized with 0.2 ml of saline containing 25 mg/ml ketamine hydrochloride and 2.5 mg/ml xylazine. The animals were moved to a Kopf stereotactic head frame (David Kopf Instruments). With the aid of an operating microscope, a 2-mm burr hole was made approximately 2 mm lateral to the midline and 1.5 mm posterior to the lambdoid suture. A 10 μl, 26-gauge Hamilton Gastight 1701 syringe needle (Sigma-Aldrich) was inserted to a depth of 4 mm. Over a period of 4 min, 5 μl of the 5×105/ml U87 cell suspension was injected in the striatum using a multiport Microinfusion Syringe Pump (Harvard Apparatus Inc.).
Treatment of glioma model
For tumour targeting experiments, 24 mice that received glioma cell implants were randomly assigned to the PBS group (n=8), the MSC group (n=8) or the PEDF-engineered MSC group (n=8). After 7 days, when the tumours were well established, 106 cells in 200 μl of DMEM of PBS, MSC or MSC–PEDF were injected into the tail vein of xenograft-bearing nude mice every 2 days for the duration of the experiment.
Immunohistochemical staining
Murine brains were removed and frozen in solid CO2, sectioned using a cryostat, mounted on slides and allowed to air dry. Sections are fixed with 0.05% glutaraldehyde, stained as the standard protocol and counterstained with Neutral Red before mounting. For other stains, brain sections are fixed in acetone and stained using primary antibodies for CD34 (1:200 dilution; DAKO). Secondary staining was then performed with goat anti-mouse IgG antibody conjugated with rhodamine isothiocyanate (1:800 dilution; Upstate Biotechnology). Slides were counterstained with DAPI (4´,6-diamidino-2-phenylindole) before the final mounting. MVD (microvessel density) was assessed according to the method of Weidner et al. [15].
Statistical analysis
Statistical analysis for the cell proliferation assays and the migration assay was performed by Student's t-test. Survival was measured from the day of U87 cell injection to the day of death. Survival data were analysed using the log rank test. A P-value<0.05 was considered statistically significant. The analysis was carried out using SPSS 13.0.
RESULTS
Characterization of hMSCs
We analysed the surface antigens on hMSCs by flow cytometry. Although hMSCs do not have a specific antigen profile, we identified that cultured hMSCs are positive for the mesenchymal markers CD44, CD105 and CD166. MSCs are negative for typical haematopoietic antigens CD45, CD34 and CD11b, indicating no contamination with haematopoietic cells from the bone marrow (results not shown).
Tropism of hMSCs towards glioma cells
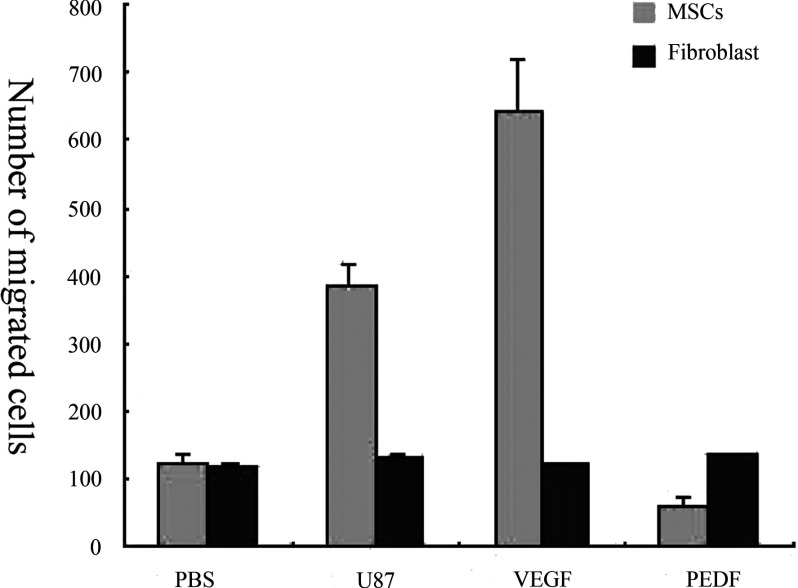
The migratory capacity of hMSCs towards glioma cells was evaluated in vitro. At 48 h later, most of the EGFP-labelled hMSCs aggregated around the U87 clones of glioma cells, whereas human fibroblast cells merely plated at any other place (results not shown). To further quantitatively evaluate the migratory pattern of hMSCs towards glioma cells, EGFP-labelled hMSCs were cultured in the upper chamber and the target cells were plated in the lower chamber. After co-culturing for 48 h, we counted EGFP-labelled MSCs that had migrated to the lower chamber. It showed that saline did not have any effect on the migration of either MSCs or fibroblast cells in vitro. In contrast, VEGF significantly stimulated migration of MSCs. Migratory activity was significantly inhibited with the presence of PEDF (P<0.05, Figure 1). The data indicated that angiogenic factors present in the U87 media were capable of attracting or inhibiting the migration of hMSCs towards the U87 cells.
Figure 1. Migration pattern of MSCs and fibroblast in vitro.
Compared with the PBS, U87cells and VEGF led to a distinct increase of MSCs migration. The PEDF, however, showed an inhibitory effect on MSCs. Compared with fibroblasts, MSCs possess greater migratory capacity than fibroblasts under the stimulation of VEGF or cultured U87 cells (*P<0.05 compared with the PBS group, Student's t test). The values are means±S.E.M for three independent experiments.
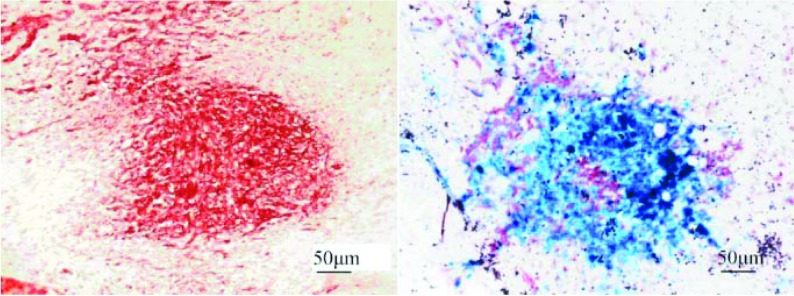
To further evaluate the migration of hMSCs in vivo, intracranial xenografts of human glioma were established in the right caudate nucleus and subsequently LacZ-labelled hMSCs were injected intravenously 7 days later. The animals were killed 7 days after injection, the brain sections were analysed by microscopy. It showed that LacZ-labelled MSCs infiltrated into the bed and scattered throughout the tumour 7 days after injection (Figure 2). These results confirmed the capacity of hMSCs to migrate towards glioma after intracranial transplantation.
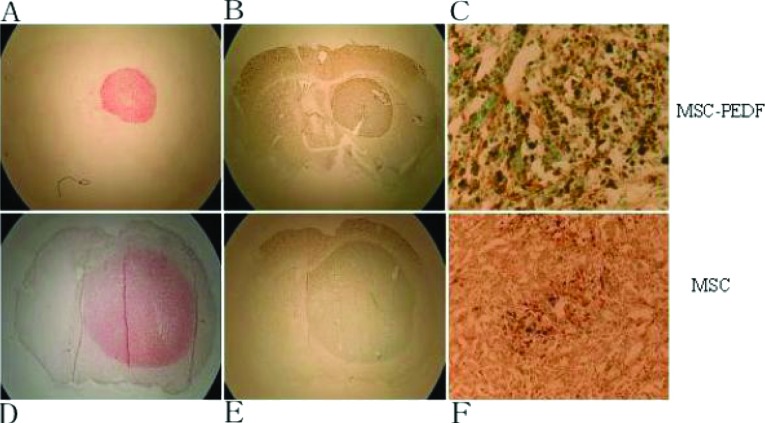
Figure 2. Photomicrographs of LacZ-labelled MSCs injected intravenously after transplantation.
The sections were co-stained with X-Gal (5-bromo-4-chloroindol-3-yl β-D-galactopyranoside) (allowing the LacZ-expressing MSCs to stain blue) and with Neutral Red (allowing the elongated glioblastoma cells to stain dark red. X-Gal staining showed a large number of LacZ-labelled MSCs aggregated in the tumour bed (right-hand panel, ×40). The blue MSCs infiltrated into the bed and scattered throughout the tumour 7 days after injection.
AAV–PEDF successfully transferred the PEDF gene into hMSCs
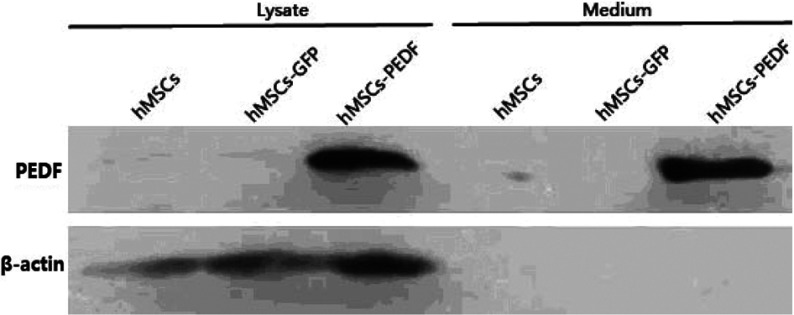
The success of AAV-mediated gene transfer mainly depends on its ability to infect target cells and express the recombinant gene. Therefore we first tested whether recombinant AAV–PEDF virus is capable of infecting hMSCs, which then can express PEDF protein in vitro. hMSCs were infected with AAV–PEDF, and the supernatant of the media was prepared and subjected to Western blot analysis. PEDF was found in the cells after they had been infected for 24 h, with the expression level elevating as the incubating duration prolonged (results not shown). As shown in Figure 3, PEDF was detected in the supernatant from the media of MSC–PEDF cells infected for 24 h. These results show that AAV–PEDF is stably expressed in infected hMSCs, and MSC–PEDF secretes some PEDF to the medium.
Figure 3. Western blot analysis of AAV–PEDF-transduced hMSCs, conditional medium from hMSC–PEDF cells infected for 24 h.
Immunoblot analysis of the lysates shows MSC–PEDF protein (46 kDa) expression in AAV–PEDF-transduced MSCs. Except for the intracellular expression, hMSC–PEDF can also be secreted as a soluble protein by the infected cells into the culture medium. β-Actin was used as a loading control.
MSC–PEDF cells prolong the survival of tumourbearing mice
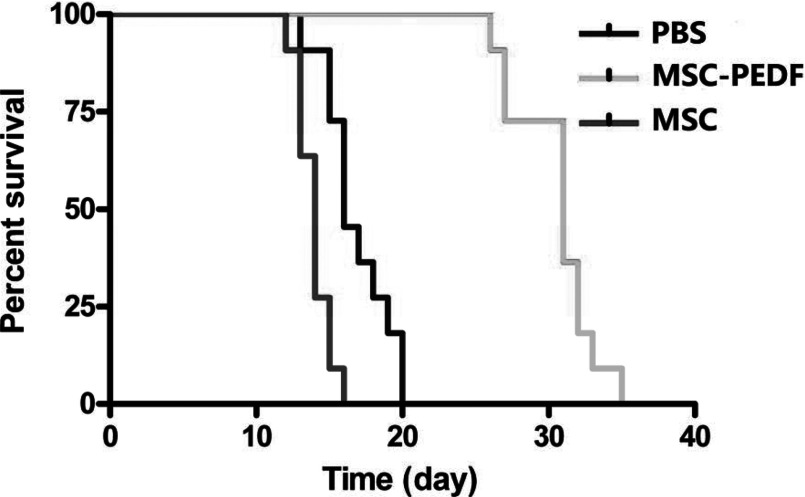
PEDF was selected as a therapeutic gene because of the anti-tumour effect of PEDF on brain tumour [16]. In order to assess the feasibility of PEDF delivery by MSCs to glioma in vivo, we used mice bearing intracranial glioma xenografts. After tumour growth for 7 days, PBS, MSC (106 cells) or MSC–PEDF (106 cells) were injected through the tail vein. The mice were monitored until death, and the survival times were compared with the treatment groups by using the log-rank test (Figure 4).
Figure 4. Therapeutic effect of MSC–PEDF against intracranical U87MG glioma.
To assess the therapeutic effect of MSC–PEDF for intracranical U87MG glioma, mice were intravenously injected with MSC–PEDF 7 days after intracranial implantation of tumour cells, and intravenous injection of MSC or PBS as control groups. Intravenous injection of MSC–PEDF has more benefits on the survival of tumour-bearing mice than PBS and MSC–LacZ-treated groups (P<0.01).
The survival of mice injected with MSC–PEDF (36.13±10.52 days, P=0.009) was significantly longer compared with those injected with PBS or unmodified MSCs (20.87±1.96, 18.12±3.95 days, respectively), but there was no difference between PBS and MSC group survival (P > 0.05).
MSC–PEDF-induced apoptosis and inhibited angiogenesis in tumour tissue
With the observation of prolonged survival after MSC–PEDF treatment, it is important to clarify if PEDF indeed acts on glioma tissue and causes histological change. To address this, we measured the apoptosis of glioma tissue using TUNEL staining. As shown in Figure 5, MSC–PEDF tumours demonstrated cellular apoptosis in tumour mass (C and E), whereas tumour-bearing brain sections from animals treated with MSC show negligible TUNEL staining of the tumour (D and F). TUNEL fragmented DNA can be detected within apoptotic glioma cell nuclei. Furthermore, apoptosis is localized in the glioma tissues, which suggests that the growth inhibition of glioma with MSC–PEDF treatment may be at least partially caused by increased apoptosis.
Figure 5. Immunohistochemical detection of apoptosis in MSC–PEDF-treated gliomas.
Immunohistochemistry was used to detect apoptosis in MSC–PEDF-treated gliomas. MSC or MSC–PEDF was intravenously injected in mice with U87MG gliomas. After 15 days, the treated brains were harvested and stained for TUNEL. MSC–PEDF-treated tumours were stained with TUNEL, demonstrating cellular apoptosis in tumour mass (C, E), whereas tumour-bearing brain sections from animals treated with MSC show negligible TUNEL staining of the tumour (D, F). (A) and (B) were stained with Neutral Red; (C) and (D) stained with TUNEL (slides were developed with DAB and counterstained with Methyl Green); (E) and (F) are shown at higher magnifications than in (C) and (D), respectively. Scale bar, 120 μm.
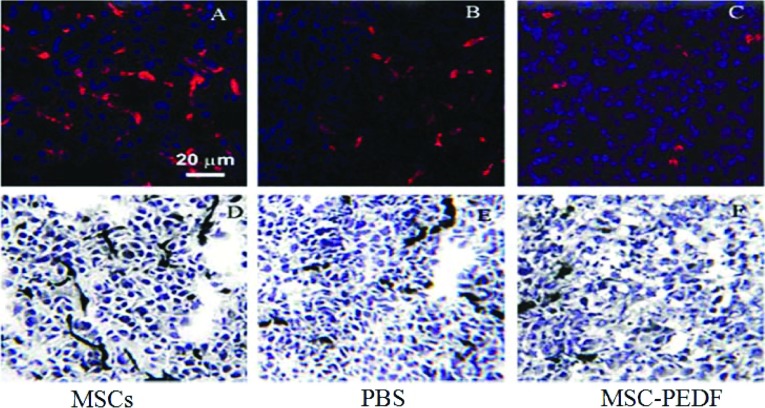
To further determine whether the increase in apoptosis of PEDF-treated glioma tissue was associated with its anti-angiogenic effect, we measured the MVD of glioma tissues in three groups. As shown in Figure 6, intensive CD34 immunoreactive microvessels were observed in glioma tissue from mice treated by PBS or MSCs, but only moderate CD34 staining was present in tumour tissue from mice-treated with MSC–PEDF. MVD of tumour tissues from MSC–PEDF-treated mice exhibited a marked reduction (30.5±7.1) than from MSCs or PBS-treated mice, (65.3±4.9, 51.8±4.8, respectively) (P<0.05). The anti-tumour activity of MSC–PEDF may be bound up with destruction of the tumour vascular network. These data suggest that MSC–PEDF treatment decreased angiogenesis, which may explain in part the increased apoptosis.
Figure 6. MSC–PEDF administration is associated with decreased tumour angiogenesis.
Glioma vascularization was determined by staining with anti-CD34 antibody after MSC or MSC–PEDF treatment. Decreased density of vessels was observed in tumours from MSC–PEDF-treated mice (C) (*P<0.05). In contrast, a mass of vessels were seen in MSCs (A) and PBS groups (B). (Original magnification ×200)
DISCUSSION
Initial results from our group showed the anti-angiogenic and therapeutic properties of PEDF to gliomas [17]. However, lack of the effective vector delivering the therapeutic gene PEDF may constitute the obstacle for glioma gene therapy. In the present study, we demonstrated the prolonged survival and anti-angiogenic effects mediated by intravenous administration of the PEDF gene-modified MSCs in the glioma model and MSC–PEDF did affect tumour MVD and promote apoptosis of glioma-bearing mice.
Gliomas are highly aggressive tumours and are characterized by marked angiogenesis and extensive invasion into the normal brain parenchyma [18]. Anti-angiogenesis has been proposed as a therapeutic strategy for cancer treatment since the 1970s, but it has been limited by the unavailability of angiogenic inhibitors and inefficient administration modes. In the past two decades, several angiogenic inhibitors, such as angiostatin, thrombospondin and PEDF, have been found and characterized. Many studies have shown that the tumour vasculature can be a selective target without affecting the existing vessels [19,20]. Anti-angiogenic therapy of gliomas and of brain metastases have previously shown that the systemic anti-angiogenic inhibition causes decreased tumour vascularity as well as a marked increase in tumour cell apoptosis in intracranial tumours [21,22].
Stem cells have been explored as vehicles for gene therapy in brain tumour because they migrate towards tumour cells. MSCs can be easily isolated from the bone marrow and migrate after local intracranial delivery to the glioma [8]. In our in vitro study, the migratory capacity of hMSCs capacity was enhanced as compared with control fibroblasts, and the tropism of hMSCs for gliomas may be mediated in part by specific growth/inhibitory factors. In vivo assays have further revealed that MSCs mainly scatter throughout the glioma, and some hMSCs penetrate the vessels surrounding the tumour. These results indicate that the hMSCs can specifically migrate to gliomas.
PEDF, a 50-kDa glycoprotein and a member of the serine protease inhibitor gene family, was initially confirmed as a potential neurotrophic and neuroprotective factor that promotes the survival of cerebellar granule cells as well as spinal motor neurons from damage caused by increased intraocular pressure of transient ischaemic reperfusion [23]. Recently, it has been shown that PEDF potently inhibited endothelium cell migration in a dose-dependent manner, placing it among the most potent natural inhibitors of angiogenesis [24]. Since PEDF selectively and potently suppresses new vessel growth with least impact on the pre-existing vessels, it is one of the top candidates for tumour therapy. In addition, PEDF can completely block GM-CSF (granulocyte/macrophage colony-stimulation factor)-stimulated cell division of microglia in rats, which is called the ‘gliastatic’ effect [25]. These data indicated that PEDF may function as a promising candidate for gene therapy of glioma. The mechanism of PEDF inhibition of glioma migration in vivo is unknown. However, it has been demonstrated that MSCs are capable of differentiating into glial cells [9,26], including astrocytes, and it is thus possible that PEDF inhibited the migration of MSC accompanied by impaired homing of MSCs to gliomas.
Previous results showed that MSCs localized to gliomas were of interest because they indicated that the capacity of integration into tumour is an intrinsic property of these stem cells [8,9]. Therefore MSCs may act as very potent vehicles for the delivery of anti-angiogeneic gene when designing anti-angiogeneic clinical trails for gliomas. Although the exact molecular mechanism of PEDF is not fully illustrated yet, a number of researches show that VEGF, MAPK (mitogen-activated protein kinase) and PPARγ (peroxisome-proliferator-activated receptor γ) may be the key mediators in the complex process of glioma angiogenesis. Meanwhile, PEDF can activate the Fas–caspase cascade to induce the activation of apoptotic signalling pathway [11,27–29]. It is worth noting that Daniel et al. [30] reported that MSCs grafting did not affect tumour MVD and survival of glioma bearing animals. Our experiments indicated a profound inhibition of glioma cells and prolonged survival of glioma-bearing mice treated with MSC–PEDF. Suppression of glioma growth was associated with increased apoptosis and was characterized by a low number of blood vessels. When injected into the animal glioma model, MSC–PEDF might be integrated into the host vessels and thus decreased the angiogenesis, with a consequent reduced blood supply, which was pivotal to glioma growth and metastasis.
Future studies should focus on the molecular mechanism by which PEDF causes gliomas apoptosis and anti-angiogenesis. The dodgy problem on the way to fully understanding the signalling pathways involved in the anti-tumour activity of PEDF is the question of existence of a putative PEDF receptor, which is still obscure [31]. To further verify the exact mechanism of PEDF in the gliomas, we need to explore the Fas–caspase and p53 pathway in the PEDF gene-modified MSCs.
Taken together, the findings of our study suggest that PEDF-engineered MSCs act as an inhibitory molecular vehicle to promote apoptosis and attenuate angiogenesis in gliomas and thus they may have potential as a therapeutic agent in the clinical application of stem cell therapy against gliomas.
AUTHOR CONTRIBUTION
All authors had full access to the study data and shared responsibility for the final decision to submit for publication. Study concept and design: Qiaoshu Wang, Zi Chen and Tao Zhang. Analysis and interpretation of the data: Tianling Ding. Drafting of the paper: Tao Zhang. Critical revision of the paper for important intellectual content: Zhaoyun Zhang. Statistical analysis: Qiaoshu Wang and Zi Chen.
FUNDING
This work was supported by the National Clinical Key Subject, the National Natural Science Foundation of China [grant number 81072070], the Natural Science Foundation of Shanghai [grant number 12ZR1424400], the the Ph.D. Programme Foundation (to young teachers) of the Ministry of Education of China [grant number 20070246067], the Shanghai Municipal Health Bureau [grant numberXYQ2011002] and the Shanghai Committee of Science and Technology [grant number 11PJ140000].
References
- 1.Folkman J. Fundamental concepts of the angiogenic process. Curr. Mol. Med. 2003;3:643–651. doi: 10.2174/1566524033479465. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 3.Donato V., Papaleo A., Castrichino A., Banelli E., Giangaspero F., Salvati M., Delfini R. Prognostic implication of clinical and pathologic features in patients with glioblastoma multiform treated with concomitant radiation plus temozolomide. Tumori. 2007;93:248–256. doi: 10.1177/030089160709300304. [DOI] [PubMed] [Google Scholar]
- 4.Kang S. G., Kim J. H., Nam D. H., Park K. Clinical and radiological prognostic factors of anaplastic oligodendroglioma treated by combined therapy. Neurol. Med. Chir. 2005;45:232–238. doi: 10.2176/nmc.45.232. [DOI] [PubMed] [Google Scholar]
- 5.Jansen M., de Witt Hamer P. C., Witmer A. N., Troost D., van Noorden C. J. Current perspectives on antiangiogenesis strategies in the treatment of malignant Gliomas. Brain Res. Brain Res. Rev. 2004;45:143–163. doi: 10.1016/j.brainresrev.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Peterson B. E., Bowen W. C., Patrene K. D., Mars W. M., Sullivan A. K., Murase N., Boggs S. S., Greenberger J. S., Goff J. P. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Ramos J. R. Neural cells derived from adult bone marrow and umbilical cord blood. J. Neurosci. Res. 2002;69:880–893. doi: 10.1002/jnr.10337. [DOI] [PubMed] [Google Scholar]
- 8.Nakamizo A., Marini F., Amano T., Khan A., Studeny M., Gumin J., Chen J., Hentschel S., Vecil G., Dembinski J., et al. Human bone marrow derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura K., Ito Y., Kawano Y., Kurozumi K., Kobune M., Tsuda H., Bizen A., Honmou O., Niitsu Y., Hamada H. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene. Ther. 2004;11:1155–1164. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 10.Ren J. G., Jie C., Talbot C. How PEDF prevents angiogenesis: a hypothesized pathway. Med. Hypotheses. 2005;64:74–78. doi: 10.1016/j.mehy.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Volpert O. V., Zaichuk T., Zhou W., Reiher F., Ferguson T. A., Stuart P. M., Amin M., Bouck N. P. Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factor. Nat. Med. 2002;8:349–357. doi: 10.1038/nm0402-349. [DOI] [PubMed] [Google Scholar]
- 12.Zhang T., Guan M., Lu Y. Production of active pigment epithelium-derived factor in E. coli. Biotechnol. Lett. 2005;27:403–407. doi: 10.1007/s10529-005-1549-8. [DOI] [PubMed] [Google Scholar]
- 13.Wu X., Hu J., Zhou L., Mao Y., Yang B., Gao L., Xie R., Xu F., Zhang D., Liu J., Zhu J. In vivo tracking of superparamagnetic iron oxide nanoparticle-labeled mesenchymal stem cell tropism to malignant gliomas using magnetic resonance imaging. Laboratory investigation. J. Neurosurg. 2008;108:320–329. doi: 10.3171/JNS/2008/108/2/0320. [DOI] [PubMed] [Google Scholar]
- 14.Wang H., Chen T., Ding T., Zhu P., Xu X., Yu L., Xie Y. Adipogenic differentiation alters the immunoregulatory property of mesenchymal stem cells through BAFF secretion. Hematology. 2011;16:313–323. doi: 10.1179/102453311X13085644679944. [DOI] [PubMed] [Google Scholar]
- 15.Weidner N., Semple J. P., Welch W. R., Folkman J. Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N. Engl. J. Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 16.Salsano E., Pollo B., Eoli M., Giordana M. T., Finocchiaro G. Expression of MATH1, a marker of cerebellar granule cell progenitors, identifies different medulloblastoma sub-types. Neurosci. Lett. 2004;37:180–185. doi: 10.1016/j.neulet.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 17.Zhang T., Guan M., Xu C., Chen Y., Lu Y. Pigment epithelium-derived factor inhibits glioma cell growth in vitro and in vivo. Life Sci. 2007;81:1256–1263. doi: 10.1016/j.lfs.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 18.Ulrich T. A., de Juan Pardo E. M., Kumar S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 2009;69:4167–4174. doi: 10.1158/0008-5472.CAN-08-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naumov G. N., Akslen L. A., Folkman J. Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle. 2006;5:1779–1787. doi: 10.4161/cc.5.16.3018. [DOI] [PubMed] [Google Scholar]
- 20.Neri D., Bicknell R. Tumor vascular targeting. Nat. Rev. Cancer. 2005;5:436–446. doi: 10.1038/nrc1627. [DOI] [PubMed] [Google Scholar]
- 21.Kusters B., Leenders W. P., Wesseling P., Smits D., Verrijp K., Ruiter D. J., Peters J. P., van Der Kogel A. J., de Waal R. M. Vascular endothelial growth factor-A(165) induces progression of melanoma brain metastases without induction of sprouting angiogenesis. Cancer Res. 2002;62:341–345. [PubMed] [Google Scholar]
- 22.Rubenstein J. L., Kim J., Ozawa T., Zhang M., Westphal M., Deen D. F., Shuman M. A. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2:306–314. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conway E. M., Collen D., Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc. Res. 2001;49:507–521. doi: 10.1016/s0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- 24.Dawson D. W., Volpert O. V., Gillis P., Crawford S. E., Xu H., Benedict W., Bouck N. P. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 25.Ek E. T., Dass C. R., Choong P. F. Pigment epithelium-derived factor: a multimodal tumor inhibitor. Mol. Cancer Ther. 2006;5:1641–1646. doi: 10.1158/1535-7163.MCT-06-0107. [DOI] [PubMed] [Google Scholar]
- 26.Woodbury D., Schwarz E. J., Prockop D. J., Black I. B. Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 27.Laytragoon-Lewin N. Programmed cell death: the influence of CD40, CD95 (Fas or Apo-I) and their ligands. Med. Oncol. 1998;15:15–19. doi: 10.1007/BF02787339. [DOI] [PubMed] [Google Scholar]
- 28.Kargiotis O., Rao J., Kyritsis A. Mechanisms of angiogenesis in gliomas. J. Neurooncol. 2006;78:281–293. doi: 10.1007/s11060-005-9097-6. [DOI] [PubMed] [Google Scholar]
- 29.Ho T. C., Chen S. L., Yang Y. C., Liao C. L., Cheng H. C., Tsao Y. P. PEDF induces p53-mediated apoptosis through PPARγ signaling in human umbilical vein endothelial cells. Cardiovasc. Res. 2007;76:213–223. doi: 10.1016/j.cardiores.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 30.Daniel B., Gunnarsson S., Tormin A., Darabi A., Gisselsson D., Roybon L., Scheding S., Bengzon J. Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol. Ther. 2009;17:183–190. doi: 10.1038/mt.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konson A., Pradeep S., Seger R. Phosphomimetic mutants of pigment epithelium-derived factor with enhanced antiangiogenic activity as potent anticancer agents. Cancer Res. 2010;70:6247–6257. doi: 10.1158/0008-5472.CAN-10-0434. [DOI] [PubMed] [Google Scholar]