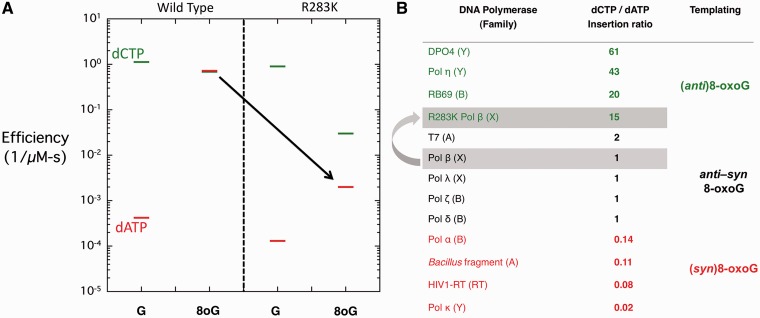
Figure 2.
Steady-state kinetic analysis of wild-type and R283K pol β. (A) A discrimination plot of 1-nt gap filling by wild-type and R283K pol β opposite non-damaged guanine (G) and 8-oxoG (8oG) with dCTP or dATP in the presence of MnCl2. The dCTP and dATP incorporation is shown with a green and red bar, respectively. The black arrow highlights the impact of the R283K mutation on dATP incorporation opposite 8-oxoG. (B) Table of select DNA polymerases (polymerase family) that have been characterized kinetically for incorporation opposite 8-oxoG (6,18,19,20–32). The dCTP/dATP ratio shows the preference for incorporation of dCTP relative to dATP. A value of 1 indicates no preference of incorporation, a value >1 indicates a preference for dCTP and a value <1 indicates a preference for dATP incorporation. The likely preferred glycosidic conformation of the templating 8-oxoG is indicated.