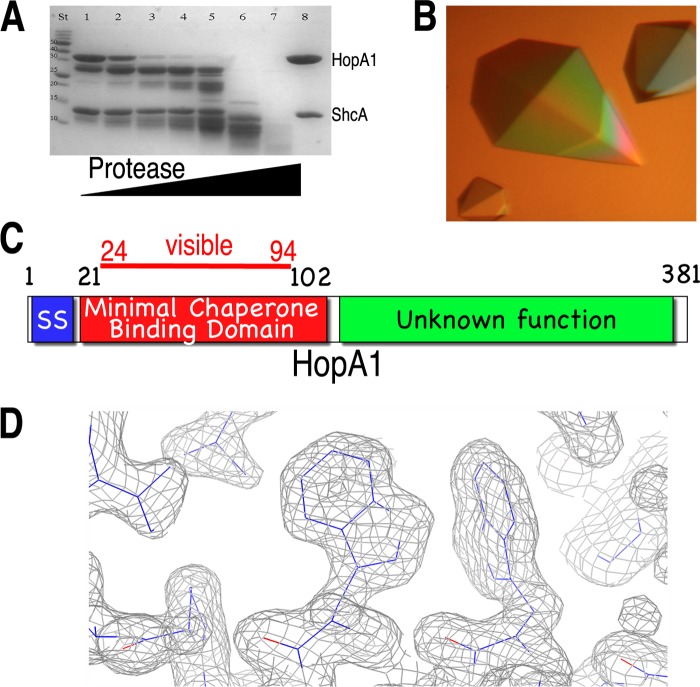
Fig 1.
Identification of the chaperone-binding domain of HopA1. (A) SDS-PAGE analysis (with Coomassie blue staining) of limited proteolytic digestion of the HopA1-ShcA complex. Lane St, protein standard markers for assigning molecular weight; lane 8, undigested purified complex of full-length proteins. For lanes 1 to 7, increasing amounts of protease were added to the reaction mixture (subtilisin/protein [wt/wt], for lanes 1 to 7: 0.0001, 0.0025, 0.05, 0.1, 1, 5, and 10%, respectively). (B) Well-diffracting crystals of the HopA1(21-102)-ShcA complex. (C) Domain delineation of the effector HopA1. SS, secretion signal. (D) Final refined (2Fo − Fc) model-phased electron density maps contoured at 1σ (gray).