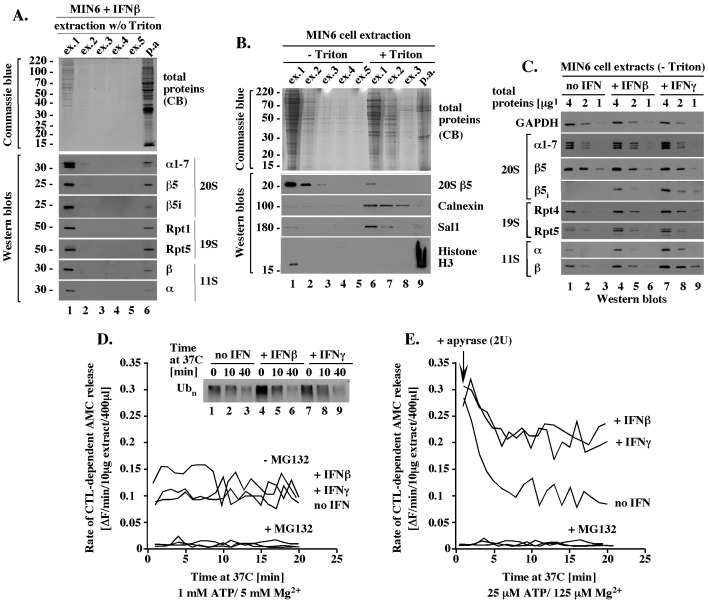
Figure 4. Functional analysis of detergent-free MIN6 cell extracts suggests that reduction in ATP levels triggers participation of the 11S in the 20S function.
(A). Rapid extraction of proteasome components with a hypotonic, detergent-free buffer. Pellet of 3×106 MIN6 cells treated with 300 U/ml of IFNβ for 24 hours was subjected to 5 sequential incubations with 2 pellet volumes (40 μl) of a hypotonic, ATP-rich, extraction buffer without detergent (ex. 1–5, -Triton; Methods) followed by solubilization of all proteins in the remaining pellet (pellet after: p.a.) by boiling with SDS-sample buffer (Methods). 12.5% of each extract was separated by SDS-PAGE and analyzed by Western blot or Commassie blue stain, as indicated. (B). The detergent-free 20S complexes are extracted together with cytosolic proteins. Experiment like in A except that the 5 sequential incubations with a detergent-free buffer (ex. 1–5, -Triton) were followed by additional 3 sequential extractions with the same buffer supplemented with 0.5% Triton (ex. 1–3,+0.5% Triton, Methods) prior to extraction of the remaining pellet with SDS sample buffer. (C). Quantitative Western blot analysis of the 20S, 19S and 11S components in detergent-free MIN6 cell extracts. Equal amounts of the total proteins (4, 2, and 1 μg) from the indicated detergent-free extracts were analyzed by Western blot with antibodies specific to the indicated proteins. (D). 20S activity in ATP-rich, detergent-free MIN6 extracts. The indicated detergent-free cell extracts with 1 mM ATP were tested in vitro for chymotrypsin-like (CTL) peptidase activity in the presence or absence of 5 μM MG132 (graph) and for degradation of polyubiquitinated proteins (Western blot insert) as described in Methods. (E). 20S activity in detergent-free MIN6 extract under condition of ATP depletion. Experiment like in D, except that the initial ATP concentration was 25 μM instead of 1 mM and apyrase (2 units) was added at time 0, to deplete ATP.