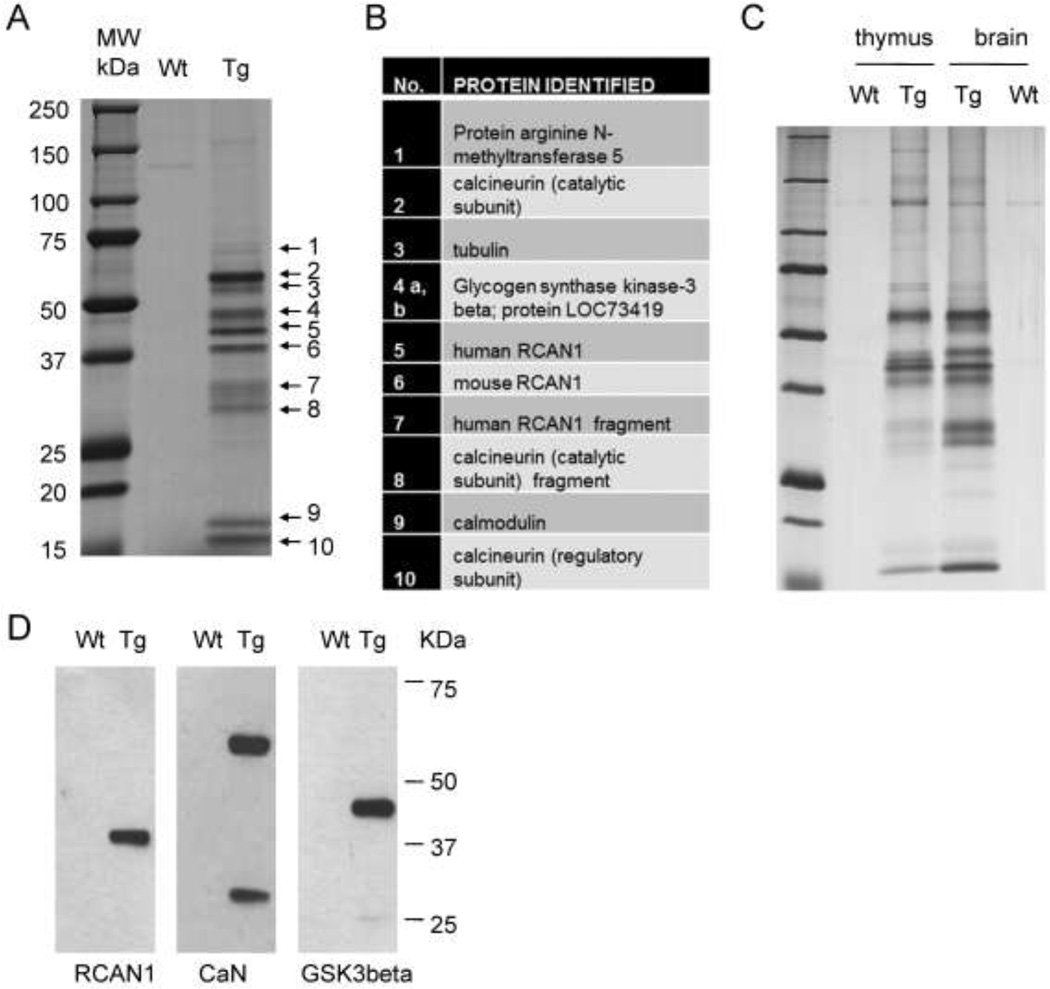
Figure 3. Purification of the RCAN1 complex from low-copy BAC-Tg mouse brain and identification of its major protein components.
A, Wild type and RCAN1BAC-Tg1 whole mouse brain lysates in hypotonic buffer were subjected to tandem affinity purification using anti-Flag- and anti-HA-beads. The final protein preparation was resolved by SDS-PAGE and individual bands were excised and analyzed by LC-MS. B, List of proteins identified in each band by LC-MS. C, Silver-stained gel of tandem affinity purified material from thymus and brain lysates, showing that the RCAN1 complex is similar in these two tissues. D, Western blots of the purified complex, independently confirming that the bait RCAN1-HA-Flag and two major interacting proteins, CaN and GSK3b, were correctly identified by LC-MS.