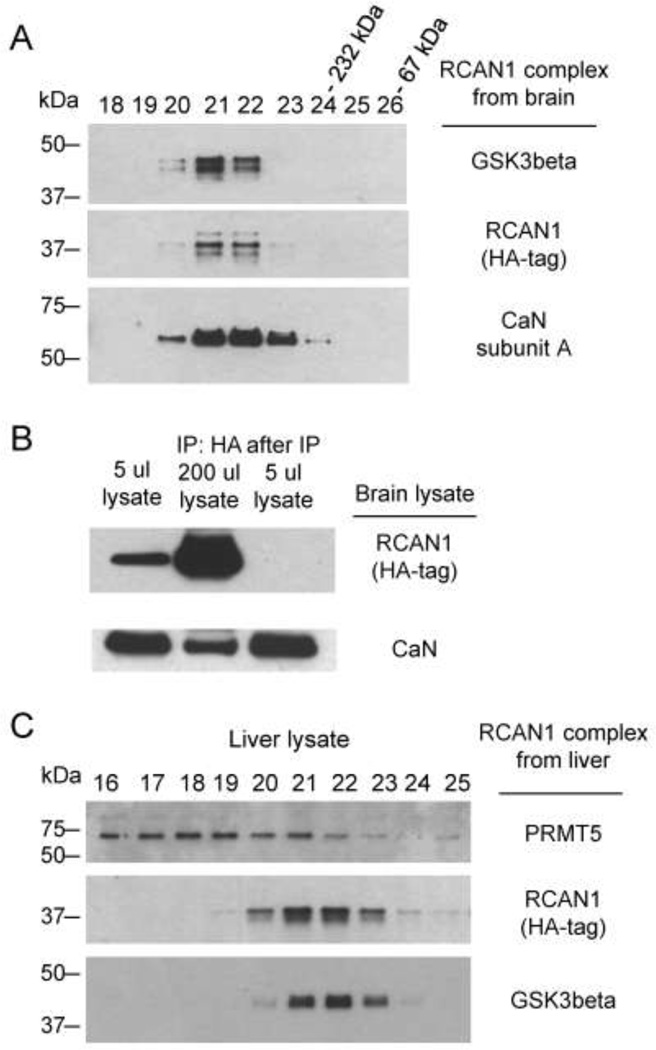
Figure 5. Stability of the interaction of RCAN1 with CaN and GSK3b.
A, CaN and GSK3b remain associated with RCAN1 through Superose chromatography of the purified complex. Based on molecular weight markers co-eluting in later fractions, the molecular weight corresponding to Superose fractions 21 and 22 is more than the sum of all these three proteins in the core complex, indicating higher order stoichiometry. B, Less than one percent of CaN associates with RCAN1 protein in whole brain lysate from RCAN1BAC-Tg1 mice. The amount of CaN in RCAN1 complex from 200 ul of lysate is compared with the CaN in 5 μl of the original lysate (2.5% input of the IP amount). C, PRMT5 is not a stable binding partner in the core RCAN1 complex. A preparation of the RCAN1 complex from RCAN1BAC-Tg1 liver lysate was loaded on the same Superose column as in (A). PRMT5, a known non-specific binder to affinity resin in HA-Flag tandem purification experiments, is not stably bound to the RCAN1 complex, as indicated by lack of co-elution.