Abstract
Background
The inferior frontal cortical (IFC)-striatal network plays an integral role in response inhibition and is compromised in patients with Bipolar Disorder (BP) or Attention-Deficit/Hyperactivity Disorder (ADHD). Prior BP functional neuroimaging studies have not accounted for ADHD comorbidity despite its high prevalence.
Methods
The authors conducted an fMRI study using a response inhibition task (Go-NoGo) in 32 euthymic adults with BP, half with comorbid ADHD (BP/ADHD); 16 adults with ADHD alone; and 30 healthy controls. Within- and between-group whole-brain analyses were performed to assess for significant neural function differences.
Results
All groups activated frontal and striatal regions involved in response inhibition. ANOVA results demonstrated significant interaction effects of BP and ADHD in the anterior and posterior cingulate, left superior and middle frontal gyri and left inferior parietal lobule. Follow-up comparisons showed significant differences between BP subjects with and without ADHD. Other regions demonstrated main effects of BP (left inferior frontal gyrus, left middle frontal gyrus, right superior frontal gyrus and left insula) and ADHD (left inferior frontal gyrus, left precentral gyrus and right anterior cingulate).
Limitations
This study, as the first of its kind, requires replication using large sample sizes and controlling for potential effects of medication.
Conclusions
Euthymic bipolar adults with comorbid ADHD have significantly different neural activation patterns from BP patients without this comorbidity. If understanding of the neurobiology of bipolar disorder is to be achieved, it is critical to control for this potential confound, something not done by most prior fMRI studies of adults with BP.
Keywords: Bipolar disorder, euthymia, fMRI, ADHD, response inhibition, inferior frontal cortex
1. Introduction
Bipolar disorder (BP) and Attention-Deficit/Hyperactivity Disorder (ADHD) are two psychiatric disorders with high prevalence and substantial comorbidity in the U.S. (Nierenberg et al., 2005, Kessler et al., 2006), with estimated rates of ADHD comorbidity in BP adults ranging from 9.5% (Nierenberg et al., 2005) to 19.4% (Kessler et al., 2006). High rates of comorbid BP and ADHD are also reported in the pediatric literature (Moreno et al., 2007), but the extent of this overlap remains unclear due to disagreements over applying BP criteria to children (Parens and Johnston, 2010). Comorbidity results in greater illness burden and complexity (McIntyre et al., 2010). In addition to high comorbidity, the two disorders share many symptoms, suggesting the possibility that similarities in neural dysfunction (specifically, impairment in IFC function during response inhibition) may be present in both disorders (Epstein et al., 2007).
Few neuroimaging studies have reported whether adults with BP have been evaluated for the presence of comorbid ADHD (Biederman et al., 2008). This is likely because studies in adults use structured diagnostic instruments of adult psychopathology [e.g., the Structured Clinical Interview for DSM-IV (SCID) (Spitzer et al., 1996)] that do not include assessments for ADHD. Thus, the possibility exists that abnormal activation patterns attributed to bipolar disorder in fMRI studies have been confounded by neural dysfunction related to the presence of unrecognized ADHD. One MRI study (Biederman et al., 2008) found that BP adults with comorbid ADHD showed structural abnormalities that were the aggregate of those found in participants with each of the disorders alone. However, there has been no four group (control, BP, ADHD, BP/ADHD) fMRI study in adults to assess whether the comorbid condition results in a sum of the neural patterns of each disorder (additive) or whether the diagnoses interact.
Bipolar disorder is associated with impulsivity during manic and euthymic mood states (Swann et al., 2001, Swann et al., 2008). Such behavioral traits suggest dysfunction in neural regions involved in response inhibition. Adults with ADHD also show increased impulsivity and demonstrate neural impairments (hypoactivation) in the inferior PFC, anterior cingulate and striatum (Cubillo and Rubia, 2010). fMRI studies examining euthymic BP adults during response inhibition tasks have produced conflicting results (Wessa et al., 2007, Kaladjian et al., 2009a). One study found no significant differences (Wessa et al., 2007) between euthymic BP and control participants, while other studies have found decreased activation in similar regions paralleling what has been reported in the ADHD literature (Blumberg et al., 2003, Strakowski et al., 2005, Kronhaus et al., 2006, Pompei et al., 2011). None of the BP studies, however, evaluated participants for the presence of comorbid ADHD. This potential confound may explain some of the conflicting results in the existing adult BP literature.
In the current study we sought to determine whether the joint effects of BP and comorbid ADHD on neural dysfunction represent a simple aggregate of the effects of each disorder (additivity) or whether the combination of the disorders results in a unique neural profile (interaction). We performed separate analyses for each of the 4 groups (control, BP, ADHD and BP with comorbid ADHD), as well as across-group comparisons. In particular we hypothesized that 1) significant deficits in bipolar subjects compared to controls in fronto-striatal regions would exist, but that 2) in some of these regions the patterns of activation would differ significantly between BP subjects with and without comorbid ADHD.
2. Methods
2.1 Participants
This protocol was approved by the institutional review boards at the University of California, Los Angeles (UCLA) and at the Department of Veterans Affairs (VA) Greater Los Angeles Healthcare System, and each participant gave written informed consent. All participants were interviewed using the Structured Clinical Interview for DSM-IV (SCID) (Spitzer et al., 1996) to determine the presence or absence of Bipolar I Disorder. Next, all participants were assessed for ADHD using the behavioral disorders module of the Kiddie Schedule of Affective Disorders (K-SADS), modified for use in adult populations (McGough et al., 2005). BP/ADHD and ADHD participants were required to meet full DSM-IV ADHD child criteria. Participants with ADHD and/or BP were excluded if they met criteria for any other current Axis I disorder. Control participants were excluded if they had current or past psychiatric diagnosis or were taking any medications for chronic medical conditions. Additional exclusion criteria for all participants were left-handedness, hypertension, neurological illness, metal implants, and a history of head trauma with loss of consciousness > 5 minutes.
Mood symptoms were evaluated in all participants on the scan day, using the Young Mania Rating Scale (YMRS) and the Hamilton Depression Rating Scale (HDRS). All subjects were required to meet the criteria for euthymic mood state by the rating scales above, namely YMRS score of ≤ 7 and a HDRS ≤ 7. Bipolar subjects additionally had to have been euthymic by self report and by SCID >1 month prior to scanning.
2.2 Functional Magnetic Resonance Imaging Procedure
Patients underwent an fMRI scan on a 3-Tesla scanner (Allegra; Siemens AG, Munich, Germany). The Blood Oxygenation Level Dependent (BOLD) contrast was evaluated using a T2-weighted EPI gradient-echo pulse sequence (TR=2500ms, TE=35ms, Flip-Angle=90°, Matrix 64×64, FOV=24cm, in-plane resolution 3.75mm × 3.75mm, slice thickness= 3mm, 28 slices). EPI structural images were obtained co-planar to the functional scans (TR=5000, TE=33 ms, 3 mm thick, matrix 1282, FOV=24 cm, 28 slices).
2.3 Activation Task
We used a Go/No-Go task with a block design to measure of response inhibition; specifically, subjects inhibit responses to rare non-targets (No-Go trials) in the context of frequent targets (Go trials). This paradigm has been used successfully in bipolar subjects while manic (Altshuler et al., 2005) and depressed (Altshuler et al., 2008) and the specifics of the paradigm have been detailed in previous papers. Briefly, the Go/No-Go task, whether using a block or event-related design [for review see (Aron and Poldrack, 2005)], has been shown to robustly activate the IFC and anterior cingulate, regions implicated in response inhibition and conflict (Cabeza and Nyberg, 2000). Our implementation of the task began and ended with 30-second rest blocks, with eight alternating 30.5-second blocks of go and no-go conditions. In the Go (control) condition, subjects were supposed to press a button each time a (randomly chosen) letter appeared on the screen. In the No-Go (experimental) condition, subjects were shown either random letters or the letter “X” and had to refrain from pressing the button when an “X” was shown. Subjects saw the letter “X” in 25% of trials and the order of the appearance of the letter “X” in the experimental block was random. Within each condition, stimulus presentation lasted 0.5 seconds, with an interstimulus interval of 1.5 seconds.
2.4 Behavioral Data Analysis
Differences in the response times and accuracy of performance for each of the conditions (Go and No-Go) were assessed using a mixed effects ANOVA model (unconstrained covariance matrix), using diagnosis as a grouping variable and condition (Go vs No-Go) as a repeated measure.
2.5 fMRI Analysis
Functional images were examined for motion or spike artifacts, and scans with >1.5mm motion were excluded. FMRI data processing was carried out using FEAT (FMRI Expert Analysis Tool) Version 5.91, part of FSL 4.0 (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). Pre-processing included motion correction (Jenkinson et al., 2002), non-brain removal, spatial smoothing using a 5mm Gaussian kernel, grand-mean intensity normalization and high-pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma=65.0s).
Time-series statistical analyses were performed using FILM with local autocorrelation correction. Registration was carried out using a 2 step transformation in FLIRT (Jenkinson et al., 2002), with a 7 degree of freedom global rescale transform to register the functional to the structural image, and a 12 degree of freedom affine transform to register structural images to standard space.
Contrasts were made for the NoGo-Go comparison for each participant using FLAME (FMRIB's Local Analysis of Mixed Effects) (Worsley, 2001) and these contrasts and their variances were submitted to second-level analysis. Specifically, at each voxel we fit a 2×2 ANOVA with main effects for BP and ADHD and their interaction. This parameterization allows different means for each of the four groups (control, BP alone, ADHD alone, comorbid) while at the same time allowing easy computation of contrasts of interest. Either significant interactions or main effects of ADHD would indicate the possibility of a confound when looking for effects of BP if ADHD status is not properly accounted for. Random effects were used to account for correlations among multiple voxels within subjects. F statistic maps were used to identify regions with significant interactions and then, after masking out the interactions, to find regions with main effects. We first determined clusters of z > 2.0 and then applied a corrected cluster threshold of P = 0.05, as described in (Worsley, 2001). Follow-up contrasts (using between-group random effects analyses in FLAME (Worsley, 2001) were used to determine the patterns of pairwise group differences in the regions with significant interactions and to determine the direction of differences in regions with only main effects. To be conservative with respect to multiple testing, results for the follow-up contrasts in were considered significant at the same cluster threshold (Z>2.0, p=0.05 corrected) used to create the overall maps although the tests were performed only in regions which had already shown significant interactions.
3. Results
3.1 Participants
Twenty participants with bipolar disorder alone (BP), 19 with comorbid BP and ADHD (BP/ADHD), 19 with ADHD alone (ADHD) and 32 control participants met inclusion criteria for the study. Four BP, 3 BP/ADHD, 3 ADHD and 2 control participants were excluded from analyses due to excessive motion artifacts in their scans. Thus, the final analyses included 32 euthymic bipolar (16 BP and 16 BP/ADHD), 16 ADHD and 30 control participants.
Demographic and medication data are shown in Table 1. We compared all four groups on demographic factors to check for possible confounds. There were no significant differences in age (ANOVA F=0.10, df=74, 3, p=0.95) or gender (Fisher’s exact test p=0.66). Additionally, we checked for differences in symptom severity within the patient groups to make sure this did not confound the key comparison of BP/ADHD to BP alone subgroups. We found no difference between the two bipolar patient subgroups’ YMRS (F=0.22, df=43, 2, p=0.80) or HDRS (F=0.77, df=42,2, p=0.47) scores (Table 1). Similarly, there were no significant differences in ADHD severity between BP/ADHD and ADHD participants based on total K-SADS scores (T=1.91, df=30, p=0.07). Six (37.5%) of the 16 BP participants and 3 (18.8%) of the 16 BP/ADHD participants were medication-free at the time of scanning (Fisher’s exact test p=0.43). 15 of the 16 participants with ADHD were unmedicated at the time of scanning (93.8%).
Table 1.
Demographic characteristics of control, BP euthymic, BP/ADHD euthymic and ADHD participants.
| Control | BP | BP/ADHD | ADHD | |
|---|---|---|---|---|
| Number | 30 | 16 | 16 | 16 |
| Age (mean years ± SD) | 36.8 ± 12.6 | 38.1±12.2 | 36.4±14.1 | 35.7±10.8 |
| Gender—Male | 17 | 12 | 9 | 10 |
| Gender—Female | 13 | 4 | 7 | 6 |
| ADHD subtype | ||||
| Inattentive | 6 | 10 | ||
| Hyperactive | 5 | |||
| Combined | 3 | 6 | ||
| Not Otherwise Specified | 2 | |||
| KADS- Total | - | - | 12.9 ± 3.5 | 10.5 ± 3.5 |
| YMRS score (mean ± SD) | 0.5±0.9 | 1.1±1.6 | 1.1±1.7 | 0.8± 1.3 |
| HDRS score (mean ± SD) | 0.9±1.2 | 3.6±1.8 | 3.8±1.9 | 3.1± 2.0 |
| Illness Duration (years ± SD) | - | 19 ± 12.4 | 22.9±14.7 | - |
| Duration Euthymic (months ± SD) | - | 11.4±16.2 | 19.3±25.6 | - |
| # Prior Manic Episodes (median) | - | 2.5 | 4.5 | - |
| # Prior Depressive Episodes | ||||
| median) | - | 6 | 7 | - |
| Medication | - | |||
| Unmedicated | 6 | 3 | 14 | |
| Anticonvulsants | 6 | 9 | ||
| Antipsychotics | 7 | 10 | ||
| Antidepressants | 3 | 8 | ||
| Stimulants | 2 |
3.2 Behavioral Data
Behavioral analyses revealed no significant differences between groups on accuracy, reaction times or in errors of commission. The behavioral data are summarized in Table 2.
Table 2.
ANOVA results show a significant BP and ADHD interaction effect (cluster Z>2.0, p=0.05 corrected).
| Region | BA | x | y | z | Z Stat |
|---|---|---|---|---|---|
| Frontal Lobe | |||||
| Left MFG | 9/46 | −40 | 18 | 34 | 3.52* |
| 8 | −36 | 32 | 38 | 3.85* | |
| Left SFG | 10 | −20 | 58 | −6 | 2.43 |
| 8 | −24 | 46 | 40 | 3.34 | |
| Left Medial FG | 11 | −4 | 54 | −12 | 3.27 |
| 9 | −4 | 56 | 16 | 3.6* | |
| 8 | −12 | 34 | 50 | 3.48* | |
| Anterior Cingulate | 32 | 0 | 40 | 20 | 3.43* |
| 24 | −2 | 0 | 30 | 3.42* | |
| 24 | −10 | −18 | 34 | 3.46 | |
| Posterior Cingulate | 31 | −4 | −40 | 38 | 3.87 |
| Parietal Lobe | |||||
| Left IPL | 40 | −44 | −72 | 38 | 3.22 |
| Left SMG | 40 | −50 | −48 | 28 | 3.81 |
| 39 | −60 | −54 | 32 | 3.56* | |
| Occipital Lobe | |||||
| Left Precuneous | 19 | −24 | −66 | 38 | 3.54 |
| 7 | −2 | −68 | 38 | 3.4 | |
| Subcortical | |||||
| Left Thalamus | −6 | −20 | 2 | 3.03 | |
| Right Thalamus | 10 | −30 | 2 | 3.6* | |
| 8 | −2 | 2 | 3.53 | ||
| Left Putamen | −22 | 8 | 8 | 2.41 | |
| Left Caudate | −12 | 4 | 10 | 3.13 | |
| Left Globus Pallidus | −12 | 0 | 2 | 2.29 | |
| Left STN | −12 | −16 | −6 | 2.12 | |
| Right STN | 14 | −10 | −4 | 2.79 | |
| Left Cerebellum | −26 | −44 | −12 | 4.18 |
Indicates there is more than one local maxima cluster within a 10mm radius.
3.3 fMRI Data
3.3.1 Within Group Analysis
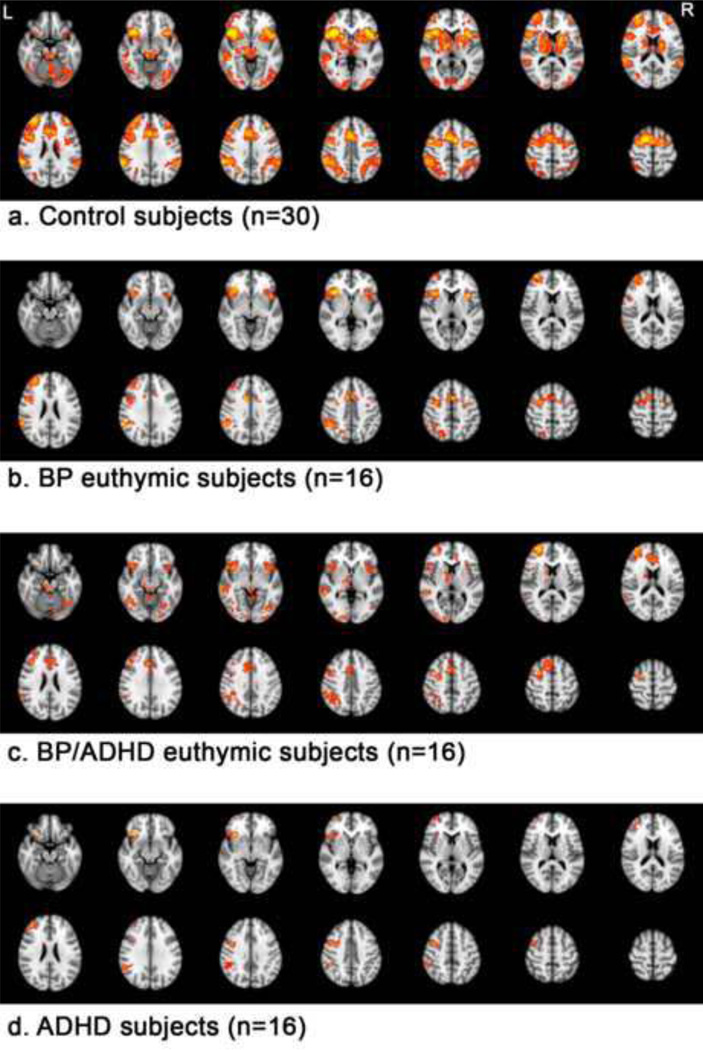
First we report results for within group analyses (NoGo-Go contrast), which were designed to examine the pattern and degree of activation in each group. Figure 1 shows the activation maps for each of the 4 groups.
Figure 1.
Within-group results are shown at Z>2.3, p=0.05 corrected, for control, BP, BP/ADHD and ADHD participants.
From top to bottom:
a. Control subjects (n=30)
b. BP euthymic subjects (n=16)
c. BP/ADHD euthymic subjects (n=16)
d. ADHD subjects (n=16)
As predicted, control participants extensively activated frontostriatal regions, including bilateral IFC (BA44/45 and 47), anterior cingulate, caudate, putamen and thalamus. Additional regions of activation included (all bilaterally) the superior frontal gyri, inferior parietal lobe, middle temporal gyrus and occipital lobe and cerebellum.
BP participants activated similar regions throughout the frontal lobe, including bilateral inferior frontal gyrus (BA47), anterior cingulate, right middle frontal gyrus (BA46/9 and BA10) and the right precentral gyrus. Other regions of significant activation included bilateral insula, right precuneus and right inferior parietal lobe. BP participants did not significantly activate striatal regions.
ADHD participants showed significant activation lateralized to the right hemisphere, including in the right inferior frontal gyrus (BA47), right middle frontal gyrus (BA46/9 and BA10), right inferior parietal lobule and supramarginal gyrus and left cerebellum.
Comorbid BP/ADHD participants showed significant activation in bilateral inferior frontal gyrus (BA47), bilateral insula, right middle frontal gyrus (BA10 and BA6), anterior cingulate, right caudate, right globus pallidus, right thalamus, right inferior parietal lobule, right middle temporal gyrus, bilateral occipital lobe and bilateral cerebellum.
3.3.2 ANOVA Interaction and Main Effects: BP/ADHD Comorbidity
To directly compare the groups, we fit a 2×2 analysis of variance (ANOVA) of neural activation at each voxel with main effects for ADHD and BP status and a BP × ADHD interaction. A significant interaction (cluster Z > 2.0, p < 0.05 corrected) would indicate that the degree of difference in neural activation patterns between bipolar and non-bipolar participants during the response inhibition task was significantly affected by whether the subject had ADHD. In regions without interactions, significant main effects would indicate differences between BP and non-BP subjects or differences between ADHD and non-ADHD subjects each without regard to the other diagnostic indicator. We note that either a significant interaction or a main effect of ADHD could imply a difference between BP/ADHD and BP alone subjects, leading to potential confounds when trying to identify deficits specifically associated with bipolar disorder.
Significant interaction effects were seen in the bilateral anterior cingulate (BA32/24), left superior and middle frontal gyri (BA8/9 and BA10), left medial frontal gyrus (BA8 and BA9), bilateral posterior cingulate (BA31), left inferior parietal lobe, left precuneus, bilateral thalamus and left striatum (Table 2). Significant main effects for BP were found in left inferior frontal gyrus (BA45/47), left middle frontal gyrus (9/46), right superior frontal gyrus (BA10), left insula and left middle and superior temporal gyrus (BA 21/22). Significant main effects for ADHD were seen in the left inferior frontal gyrus (BA45), left precentral gyrus (BA6), right anterior cingulate (BA24) and left middle and superior temporal gyrus (BA21/22).
3.3.3 Post Hoc Pairwise Comparisons
Since we were specifically interested in whether neural activation patterns in bipolar participants differed significantly by ADHD comorbidity status, we performed post hoc pairwise comparisons of BP/ADHD vs. BP subjects in regions with significant interactions. We also compared control vs. BP subjects and control vs. BP/ADHD subjects to discern the complete pattern of deficits in BP subjects relative to controls.
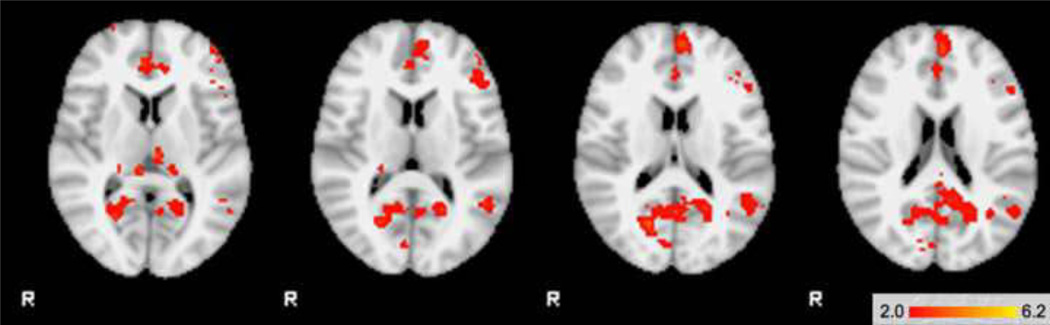
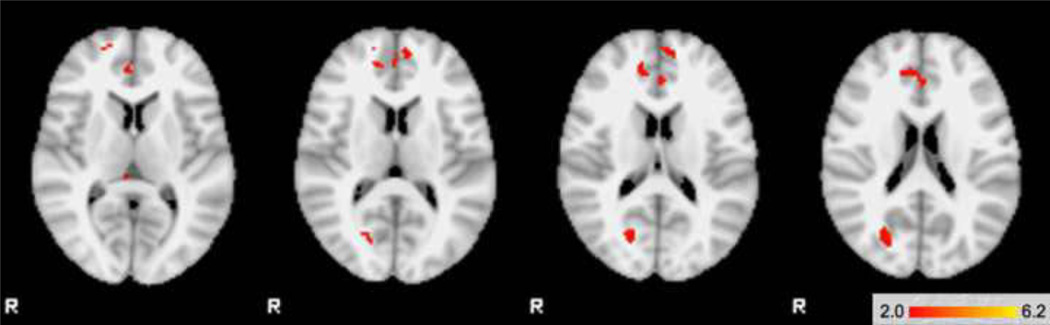
Table 3 displays the pattern of activation in areas of significant regional interaction. Within regions with significant interactions, the BP/ADHD group had significantly greater activation in anterior cingulate (BA24/32), posterior cingulate and bilateral thalamus compared to subjects with BP alone. BP/ADHD participants also showed significantly greater activation than BP participants in the left inferior frontal cortex (BA44), right superior frontal gyrus (BA10), right parahippocampal and left supramarginal gyrus (Figure 2a). There were no significant areas of activation where BP > BP/ADHD. The comorbid BP/ADHD group had significantly greater activation than control participants in the bilateral anterior cingulated (BA24/32), posterior cingulate, right thalamus, bilateral medial frontal gyrus (BA9) and left parahippocampal gyrus (Figure 2b). There were no areas where controls had significantly higher activation than comorbid BP/ADHD subjects.
Table 3.
Significant activation patterns by group*
| Regions of Significant Interaction Effects |
BP/ADHD > BP | BP/ADHD > Control |
Control > BP |
|---|---|---|---|
| Left anterior cingulate (BA24/32) | X | X | X (32) |
| Right anterior cingulate (BA24/32) | X | X | X (32) |
| Left posterior cingulate (BA31) | X | X | |
| Right posterior cingulate (BA31) | X | X | |
| Left inferior frontal cortex (BA44/45) | X | X | |
| Left frontopolar (BA10) | X | ||
| Right superior frontal gyrus (BA10) | X | ||
| Left medial frontal gyrus (BA9) | X | ||
| Right medial frontal gyrus (BA9) | X | ||
| Left dorsolateral PFC (BA9/46) | X | ||
| Left inferior parietal lobe (BA40) | X | ||
| Left putamen/caudate | X | ||
| Left thalamus | X | X | |
| Right thalamus | X | X | X |
| Left parahippocampal gyrus | X | ||
| Right parahippocampal gyrus | X |
There were no areas of BP> BP/ADHD, BP>Control or Control>BP/ADHD
Figure 2.
| TOP | 2a. BP/ADHD subjects > BP subjects |
| BOTTOM | 2b. BP/ADHD subjects > Control subjects |
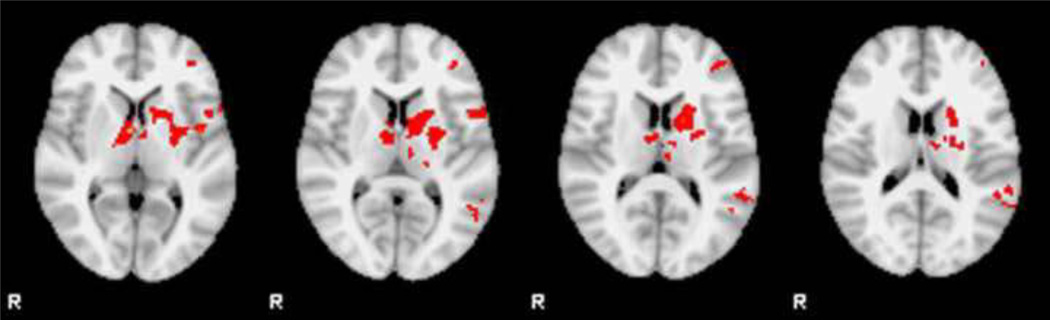
Control participants activated several areas significantly more than the participants with BP alone, including the left IFC (BA44/45), left frontopolar region (BA10), left dorsal lateral PFC (BA9/46), bilateral anterior cingulate (BA32), left parietal lobe (BA40) and numerous subcortical structures including left caudate, left putamen, right subthalamic nucleus and bilateral thalamus (Figure 3). There were no significant areas of higher activation in BP alone subjects compared to controls.
Figure 3.
Random effects results show significant differences between controls and BP participants in the IFC and striatum (control subjects > BP subjects) (cluster Z>2.0, p=0.05 corrected)
4. Discussion
Most current neuroimaging studies of adult bipolar disorder fail to consider potential confounds of comorbid ADHD, a disorder highly prevalent in patients with bipolar disorder and a disorder also associated with neural dysfunction. We found that a combined diagnosis of bipolar disorder and ADHD results in a significantly different neural activation pattern during a Go/No-Go task in multiple brain regions than a diagnoses of bipolar disorder alone. A significant interaction effect was seen in the anterior and posterior cingulate, the left medial and middle frontal gyri, the left inferior parietal lobule and precuneus, and in the thalamus and striatum. These interaction results demonstrate that the fMRI neural activation patterns seen in patients with comorbid ADHD are not simply a summation of the differences associated with each condition alone. Our comorbid BP/ADHD group showed significantly greater activation in the anterior and posterior cingulate and thalamus in comparison to both the BP and control groups. They also showed significantly greater activation in the left inferior frontal gyrus (BA44) and right superior frontal gyrus (BA10) in comparison to the bipolar only group. Most important, the controls vs. BP only results are very different than controls vs. BP/ADHD. While the control subjects had significantly greater areas of activation than the BP group in areas where there were significant interaction effects (e.g. in bilateral anterior cingulate, BA45/47 etc.), there were no areas where controls had significantly greater activation than the BP/ADHD group. This difference may in part explain inconsistent findings in the literature. Future imaging studies on subjects with BP should assess and control for potential ADHD, as the imaging findings will almost certainly be affected by the proportion of subjects with the comorbidity that are included. Grouping the BP and BP/ADHD participants together may lead to incorrect conclusions about the pathophysiology of bipolar disorder.
Specific to the task used in this study, it is possible that unaccounted-for ADHD comorbidity could help explain conflicting results found in two prior studies of response inhibition in euthymic bipolar subjects. One study using the Go/No-Go task found decreased activation in the left frontopolar region in euthymic bipolar subjects, consistent with our current results (Wessa et al., 2007). However, a second study using both emotional and nonemotional stimuli in a Go/No-Go task found no significant differences between euthymic bipolar and control participants (Kaladjian et al., 2009b). Both studies relied on the SCID for psychiatric diagnoses and thus participants likely were not assessed for the presence of ADHD. Our current results might help to explain some of these discrepancies. Given that adult ADHD studies report hypoactivation of frontostriatal regions (Cherkasova and Hechtman, 2009), we hypothesized that reduction in frontostriatal activation in published studies of bipolar disorder might be attributable to comorbid ADHD. However, direct comparison of the control and BP only participants revealed significant group differences in the frontostriatal network. A recent study of BP euthymic subjects also demonstrated a similar reduced activation and reduced functional connectivity in frontostriatal regions compared to control subjects (Pompei et al., 2011). Thus, even while controlling for ADHD comorbidity, BP subjects demonstrated significantly less frontostriatal activation during response inhibition, suggesting the existence of neural deficits specific to bipolar disorder in this brain region.
The ability to modulate or inhibit responses is generally attributed to neural activity in the IFC, as demonstrated by non-clinical controls in whom greater impulsivity is associated with attenuated IFC activation (Horn et al., 2003). Lesion studies support the role of the IFC in response inhibition, as damage to the IFC results in increased motor activity and behavioral changes including hyperactivity and disinhibition (Starkstein et al., 1988). Studies of control subjects have demonstrated the essential role of the frontostriatal network in inhibition, which includes the IFC, the anterior cingulate and the striatum (Aron, 2007, Casey et al., 2002). Reduced IFC activation in the euthymic state may be responsible for the persistent behavioral symptoms of impulsivity reported previously (Swann et al., 2008). Reduced activation in this region in euthymic bipolar subjects has also been demonstrated using interference tasks (Blumberg et al., 2003, Kronhaus et al., 2006), and as such may represent a neural phenotype of chronic underactivation of a modulatory network that regulates not only movement/activity, but also mood.
The activation patterns in the cingulate across patient and control groups are complex, reflecting the heterogeneity of this structure in terms of its anatomical connectivity and cytoarchitecture (Vogt et al., 2005). While control participants activated the anterior cingulated cortex (ACC), the area of activation was in the mid-supracallosal ACC. This region has connections to the premotor cortex and the DLPFC and has been shown to be activated during motor and error detection (Botvinick et al., 2004, Beckmann et al., 2009). Our results revealed significant interaction effects of BP and ADHD throughout the ACC, including a distinct, more anterior region of the ACC that has reciprocal connections to the orbitofrontal cortex, striatum and amygdala in both nonhuman primates (Carmichael and Price, 1994) and humans (Johansen-Berg et al., 2008, Stein et al., 2007). This area is active in emotion, reward and conflict monitoring tasks (Cabeza and Nyberg, 2000, Botvinick et al., 2004, Beckmann et al., 2009). BP/ADHD participants had significantly greater activation than both control and BP only participants in this particular ACC region. The anatomic and functional connections of the ACC to the frontal lobe and subcortical regions possibly permit it to serve a modulatory role between the two. Hyperactivity of a similar region in the ACC has been shown in major depressive disorder (Goldapple et al., 2004, Kennedy et al., 2007) and may be a marker of increased effort needing to be exerted to perform inhibitions tasks in BP subjects with comorbid ADHD. Alternatively, studies show activation of the ACC during times of emotional stress (Price, 2000, Masten et al., 2009), and this greater ACC activation may reflect the increased stress experienced by BP subjects with the additional ADHD burden when asked to perform such tasks. The other area within the cingulate that showed both a significant interaction effect and was hyperactive in BP/ADHD participants compared to BP only participants was the posterior cingulate, which has anatomical connections to the parietal lobe and hippocampus. Although the exact functional connectivity of the cingulate and its subregions is still unknown, the results of this study suggest that hyperactivity of the anterior cingulate may signal a difference in the larger fronto-limbic-striatal circuit that is essential to both response inhibition and emotion. It may be that having both ADHD and BP results in an additional load on the attentional network to complete the task at the same level of accuracy, and the neural correlate of this is increased cingulate activity.
This is the first fMRI study to examine differences between adult BP, ADHD and comorbid participants. The comorbid BP/ADHD group did not simply show the aggregate of patterns of the ADHD and BP only groups. Our results provide evidence of a possible BP/ADHD functional biosignature, beyond the aggregate of deficits seen in either condition. These results, if replicated, suggest that future neuroimaging studies need to account for ADHD comorbidity, as the comorbid condition presents additional abnormal activation patterns. This unique activation pattern compared to subjects with only BP may be useful in the future use of fMRI to aid in psychiatric diagnosis.
Our study has several limitations. First, despite this being the largest sample of euthymic bipolar participants using a response inhibition task, the samples sizes of the subset of BP only and BP/ADHD groups are modest. Second, as the control and majority of ADHD participants were unmedicated while the BP and BP/ADHD participants were largely medicated, there exists a possible confound due to medication status. However, the BP/ADHD group was not on stimulants and as such, was similar with regard to medication status to the BP only group. As the comorbid participants showed cingulate hyperactivation while the BP group showed IFC hypoactivation, it is unlikely that medication effects were solely responsible for differential activation patterns observed. This is in line with a recent meta-analysis showing the medication effects alone do not explain the main findings in the bipolar functional imaging literature (Phillips et al., 2008). Future studies using unmedicated participants and larger sample sizes could address these limitations and can help clarify the degree to which medication may or may not confound functional imaging findings.
A third potential limitation is that we used a block design, in which blocks of Go-only events were contrasted against blocks composed of both Go and No-Go events presented randomly. We specifically chose this block design to match the paradigm we had previously used in manic and depressed subjects (Altshuler et al., 2005, Altshuler et al., 2008). A block design lacks a "pure" response-withholding condition as would be seen in an event-related design; consequently the signal magnitude during the inhibition blocks might have been reduced or there may have been less power to see activity in some regions. Despite these potential limitations, we observed robust IFC activation within all groups during this task. Thus, we did not find the block design to be disadvantageous in activating response inhibition networks, consistent with Aron and Poldrack’s (Aron and Poldrack, 2005) review of block vs. event-related Go/NoGo tasks wherein both designs show robust IFC activation.
Fourth, the lack of behavioral differences precluded any meaningful behavioral correlations with functional activation. We intentionally designed the study to be easy enough for all subjects to perform adequately so as not to include any confounds related to poor behavioral performance, but this has the drawback of limiting such behavioral correlations. Similarly, there were no specific measures of impulsivity aside from the behavioral data collected. Future studies that include such metrics would help correlate clinical impulsivity deficits with functional impairments seen during fMRI. Finally, this study was focused primarily on the question of BP/ADHD comorbidity as it relates to functional deficits seen in bipolar disorder in adult population. As such, comparison of the ADHD to control and BP alone subjects and discussion of the pediatric literature were beyond the scope of this specific study.
Results from this study require further investigation and potential replication in similarly diagnosed groups, but strongly suggest that conclusions drawn from studies evaluating the underlying neural pathophysiology of bipolar disorder may not be accurate unless participants with comorbid ADHD are either excluded from or accounted for in the fMRI analyses. It is critical to control for this confound to avoid erroneous conclusions about the neural abnormalities in bipolar disorder. Future studies that examine not only neural differences in specific brain regions, but also assess how these regions may interact (connectivity studies) may provide further insight into the differences in neural patterns in BP, BP/ADHD and ADHD.
Acknowledgements
Funding Body Agreements
This study was supported by the Furlotti Family Foundation and the following two components of the National Institutes of Health (NIH): the National Institute of Mental Health [K24 MH001848 (LA), R21 MH075944 (LA), 5F31MH078556 (LF)] and the National Center for Research Resources (NCRR) (RR12169, RR13642 and RR00865).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data were presented at the Annual Meeting of the Society for Neuroscience, November 13–17, 2010, San Diego, CA
Contributors
Dr. Altshuler and Dr. McGough designed the study and wrote the protocol. Ms. Vasquez, Dr. Foland-Ross, Dr. Moody and Ms. Townsend acquired and/or preprocessed the fMRI data for each subject. Dr. Bookheimer, Dr. Altshuler, Dr. Sugar and Ms. Townsend undertook the statistical analyses. Ms. Townsend managed the literature searches and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest for Authors
Drs. Bookheimer, Foland-Ross, Moody and Sugar, Ms. Townsend and Ms. Vasquez report no financial relationships with commercial interests. Dr. Altshuler has received research support from Abbott Laboratories; consulting honoraria from Eli Lilly, Abbott Laboratories, Forest Laboratories and Sepracor; and speakers bureau honoraria from Forest Laboratories, GlaxoSmith Kline, Bristol Meyer Squibb/Otsuka America.
Dr. McGough has received consulting honoraria from Eli Lilly & Co., MedImmune, Shionogi Pharmaceuticals, Shire, Sunovion, and Targacept, and research support from Eli Lilly, NeuroSigma Inc., Supranus and Shionogi Pharmaceuticals. None of the above affiliations should result in a conflict of interest related to the subject of the manuscript being submitted.
References
- Altshuler L, Bookheimer S, Townsend J, Proenza MA, Sabb F, Mintz J, Cohen MS. Regional brain changes in bipolar I depression: a functional magnetic resonance imaging study. Bipolar Disord. 2008;10:708–717. doi: 10.1111/j.1399-5618.2008.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler L, Bookheimer SY, Townsend J, Proenza MA, Eisenberger N, Sabb F, Mintz J, Cohen MS. Blunted activation in orbitofrontal cortex during mania: a functional magnetic resonance imaging study. Biol. Psychiatry. 2005;58:763–769. doi: 10.1016/j.biopsych.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13:214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J. Neurosci. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Makris N, Valera EM, Monuteaux MC, Goldstein JM, Buka S, Boriel DL, Bandyopadhyay S, Kennedy DN, Caviness VS, Bush G, Aleardi M, Hammerness P, Faraone SV, Seidman LJ. Towards further understanding of the co-morbidity between attention deficit hyperactivity disorder and bipolar disorder: a MRI study of brain volumes. Psychol. Med. 2008;38:1045–1056. doi: 10.1017/S0033291707001791. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Leung HC, Skudlarski P, Lacadie CM, Fredericks CA, Harris BC, Charney DS, Gore JC, Krystal JH, Peterson BS. A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch. Gen. Psychiatry. 2003;60:601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J. Cogn. Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J. Comp. Neurol. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Fossella J. Clinical, imaging, lesion, and genetic approaches toward a model of cognitive control. Dev. Psychobiol. 2002;40:237–254. doi: 10.1002/dev.10030. [DOI] [PubMed] [Google Scholar]
- Cherkasova MV, Hechtman L. Neuroimaging in attention-deficit hyperactivity disorder: beyond the frontostriatal circuitry. Can. J. Psychiatry. 2009;54:651–664. doi: 10.1177/070674370905401002. [DOI] [PubMed] [Google Scholar]
- Cubillo A, Rubia K. Structural and functional brain imaging in adult attentiondeficit/ hyperactivity disorder. Expert Rev Neurother. 2010;10:603–620. doi: 10.1586/ern.10.4. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Casey BJ, Tonev ST, Davidson MC, Reiss AL, Garrett A, Hinshaw SP, Greenhill LL, Glover G, Shafritz KM, Vitolo A, Kotler LA, Jarrett MA, Spicer J. ADHD- and medication-related brain activation effects in concordantly affected parent-child dyads with ADHD. J. Child Psychol. Psychiatry. 2007;48:899–913. doi: 10.1111/j.1469-7610.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch. Gen. Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JF, Woodruff PW. Response inhibition and impulsivity: an fMRI study. Neuropsychologia. 2003;41:1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, Lozano AM, Mayberg HS. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb. Cortex. 2008;18:1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaladjian A, Jeanningros R, Azorin JM, Nazarian B, Roth M, Anton JL, Mazzola- Pomietto P. Remission from mania is associated with a decrease in amygdala activation during motor response inhibition. Bipolar Disord. 2009a;11:530–538. doi: 10.1111/j.1399-5618.2009.00722.x. [DOI] [PubMed] [Google Scholar]
- Kaladjian A, Jeanningros R, Azorin JM, Nazarian B, Roth M, Mazzola-Pomietto P. Reduced brain activation in euthymic bipolar patients during response inhibition: an event-related fMRI study. Psychiatry Res. 2009b;173:45–51. doi: 10.1016/j.pscychresns.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Konarski JZ, Segal ZV, Lau MA, Bieling PJ, Mcintyre RS, Mayberg HS. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. Am. J. Psychiatry. 2007;164:778–788. doi: 10.1176/ajp.2007.164.5.778. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K, Spencer T, Ustun TB, Walters EE, Zaslavsky AM. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am. J. Psychiatry. 2006;163:716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronhaus DM, Lawrence NS, Williams AM, Frangou S, Brammer MJ, Williams SC, Andrew CM, Phillips ML. Stroop performance in bipolar disorder: further evidence for abnormalities in the ventral prefrontal cortex. Bipolar Disord. 2006;8:28–39. doi: 10.1111/j.1399-5618.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, Mcnealy K, Mazziotta JC, Dapretto M. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc Cogn Affect Neurosci. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgough JJ, Smalley SL, Mccracken JT, Yang M, Del'homme M, Lynn DE, Loo S. Psychiatric comorbidity in adult attention deficit hyperactivity disorder: findings from multiplex families. Am. J. Psychiatry. 2005;162:1621–1627. doi: 10.1176/appi.ajp.162.9.1621. [DOI] [PubMed] [Google Scholar]
- Mcintyre RS, Kennedy SH, Soczynska JK, Nguyen HT, Bilkey TS, Woldeyohannes HO, Nathanson JA, Joshi S, Cheng JS, Benson KM, Muzina DJ. Attention-deficit/ hyperactivity disorder in adults with bipolar disorder or major depressive disorder: results from the international mood disorders collaborative project. Prim Care Companion J Clin Psychiatry. 2010;12 doi: 10.4088/PCC.09m00861gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno C, Laje G, Blanco C, Jiang H, Schmidt AB, Olfson M. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch. Gen. Psychiatry. 2007;64:1032–1039. doi: 10.1001/archpsyc.64.9.1032. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Miyahara S, Spencer T, Wisniewski SR, Otto MW, Simon N, Pollack MH, Ostacher MJ, Yan L, Siegel R, Sachs GS. Clinical and diagnostic implications of lifetime attention-deficit/hyperactivity disorder comorbidity in adults with bipolar disorder: data from the first 1000 STEP-BD participants. Biol. Psychiatry. 2005;57:1467–1473. doi: 10.1016/j.biopsych.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Parens E, Johnston J. Controversies concerning the diagnosis and treatment of bipolar disorder in children. Child Adolesc Psychiatry Ment Health. 2010;4:9. doi: 10.1186/1753-2000-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am. J. Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompei F, Jogia J, Tatarelli R, Girardi P, Rubia K, Kumari V, Frangou S. Familial and disease specific abnormalities in the neural correlates of the Stroop Task in Bipolar Disorder. Neuroimage. 2011;56:1677–1684. doi: 10.1016/j.neuroimage.2011.02.052. [DOI] [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. Structured clinical interview for DSM-IV. New York: Biometrics Research Dept., NYC Psychiatric Institute; 1996. [Google Scholar]
- Starkstein SE, Boston JD, Robinson RG. Mechanisms of mania after brain injury. 12 case reports and review of the literature. J. Nerv. Ment. Dis. 1988;176:87–100. doi: 10.1097/00005053-198802000-00004. [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, Holland SK, Mills NP, Delbello MP, Eliassen JC. Abnormal FMRI brain activation in euthymic bipolar disorder patients during a counting Stroop interference task. Am. J. Psychiatry. 2005;162:1697–1705. doi: 10.1176/appi.ajp.162.9.1697. [DOI] [PubMed] [Google Scholar]
- Swann AC, Anderson JC, Dougherty DM, Moeller FG. Measurement of inter-episode impulsivity in bipolar disorder. Psychiatry Res. 2001;101:195–197. doi: 10.1016/s0165-1781(00)00249-3. [DOI] [PubMed] [Google Scholar]
- Swann AC, Steinberg JL, Lijffijt M, Moeller FG. Impulsivity: differential relationship to depression and mania in bipolar disorder. J. Affect. Disord. 2008;106:241–248. doi: 10.1016/j.jad.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Farber NB, Bush G. Architecture and neurocytology of monkey cingulate gyrus. J. Comp. Neurol. 2005;485:218–239. doi: 10.1002/cne.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessa M, Houenou J, Paillere-Martinot ML, Berthoz S, Artiges E, Leboyer M, Martinot JL. Fronto-striatal overactivation in euthymic bipolar patients during an emotional go/nogo task. Am. J. Psychiatry. 2007;164:638–646. doi: 10.1176/ajp.2007.164.4.638. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. Ch 14. In: JEZZARD P, MATTHEWS PM, SMITH SM, editors. Functional MRI: An Introduction to Methods. New York: Oxford University Press; 2001. [Google Scholar]