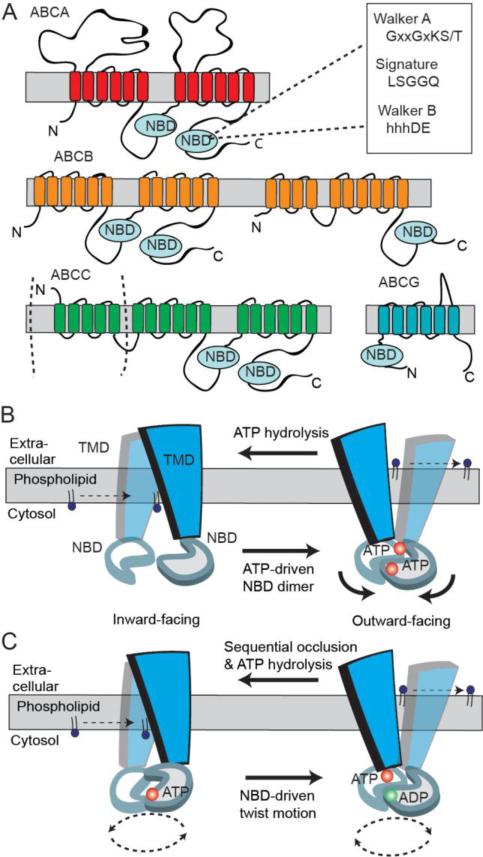
Fig 4.
Predicted two-dimensional topology of lipid ABC transporters and models of ABC transporter mechanism. A. The two transmembrane domains (TMDs) and nucleotide binding domains (NBDs) arrange to form full transporter and are represented by some ABCB members and ABCA members with additional exocytosolic domains. Some ABCC family members are full transporters with an additional N-terminal TMD. Half transporters in ABCB and ABCG subfamilies have different modular organizations of a TMD and NBD. B. A simplified‘Switch Model’: In the inward-facing conformation, the NBDs are separated and nucleotide-free. Phospholipid substrate entry into the substrate binding site occurs from the cytosolic leaflet of the membrane bilayer. ATP-dependent dimerization of NBDs progresses to pull the TMDs from an inward- to outward-facing conformation. Phospholipid is translocated to the extracellular side of the membrane and ATP hydrolysis resets the transporter in the inward conformation. C. A simplified ‘Constant-contact Model’: The two NBDs remain associated in the sandwich dimer form throughout the transport cycle. Inward and outward conformations are directed via subunit conformational changes and the twisting motion of NBDs. ATP hydrolysis alternates with one site hydrolyzing nucleotide that facilitates the binding and hydrolysis in the other site.