Abstract
Alzheimer’s disease (AD) and dementia with Lewy bodies (DLB) are common etiologies of dementia with overlapping clinical features. Our objective was to determine which extrapyramidal signs (EPS) are most helpful in identifying DLB. We analyzed data from the National Alzheimer’s Coordinating Center, including demographics, Unified Parkinson’s Disease Rating Scale (UPDRS) scores, Mini Mental State Exam (MMSE) scores, and clinical diagnosis. The subjects were divided into three groups: AD, DLB or LBV (Lewy body variant). The UPDRS motor scores were totaled and analyzed within and across MMSE strata using regression techniques. Next, we divided UPDRS subscores into 9 EPS, dichotomized as either present or absent. Logistic regression analysis was used to compare each of the EPS in the AD and LB (DLB+LBV) groups. DLB subjects (n=130) were more likely to be male, younger, and have higher MMSE scores (p<0.001) than AD (n=1,826) or LBV (n=105) subjects. Differences were found for total UPDRS score and number of EPS (p<0.001), after controlling for age, gender and MMSE. Logistic regression models demonstrated that masked facies best differentiated AD from LB (OR=6.5, p<0.001, 95% CI: 3.8–11.1). If these findings are neuropathologically validated, then the presence of specific EPS may help clinicians better differentiate AD and DLB.
Introduction
Alzheimer’s disease (AD) and dementia with Lewy bodies (DLB) are common etiologies of dementia with overlapping features. Consensus criteria for DLB, which include cognitive fluctuations, visual hallucinations and spontaneous parkinsonism, were validated in a British geropsychiatry cohort followed to autopsy1,2, with sensitivity and specificity of 83% and 95%, respectively2. Similar studies from dementia cohorts in the US demonstrated much lower sensitivities (31–32%), though specificity remained high3,4.
Differentiating DLB from AD is important, as there are important therapeutic and prognostic implications to the DLB diagnosis1,5–9. To that end, this study focuses on one of the three consensus criteria, spontaneous parkinsonism. The presence of cognitive fluctuations can be difficult to assess with high inter-rater reliability10. However, some centers have developed and validated methods which have improved the reliability of the assessment of fluctuations in DLB.11,12 Visual hallucinations have a lower prevalence in dementia-based clinics13 than in geropsychiatry-based clinics2. Parkinsonian signs can occur in both DLB and AD, particularly in the later stages14. Therefore, describing the profile of motor parkinsonism [e.g., the severity of extrapyramidal signs (EPS) and the specific EPS most commonly present] in DLB and AD by dementia stage may be helpful to clinicians in differentiating these disorders. The National Alzheimer’s Coordinating Center (NACC) database includes a large, well-characterized dementia cohort with standardized assessments of parkinsonism. Using this database, we examined the relationship between dementia severity [as measured by the Mini Mental State Exam (MMSE)15] and EPS in individuals with AD and DLB. Next, we identified key EPS that, when present, suggests Lewy body disease.
Methods
Cross-sectional data from the NACC were acquired for subjects with a primary or secondary etiological diagnosis of AD and/or DLB. The data were collected from 31 federally funded Alzheimer’s disease centers between 2005 and 2009. A full description of the Uniform Data Set has been previously published16. All centers entering data into the NACC database had institutional review board approval to gather data from human subjects. Written informed consent was obtained from all participants or their surrogate decision makers.
Participants
Subjects were included if (1) they were 65 years old or older, (2) had a clinical diagnosis of mild cognitive impairment (MCI)17 or dementia, (3) had an etiological diagnosis of probable AD18 and/or DLB1,(4) had Unified Parkinson’s Disease Rating Scale (UPDRS)19 data and (5) were not taking antipsychotic or other antidopaminergic medications. UPDRS data included only the motor subscale. Subjects were excluded if they carried a clinical diagnosis of Parkinson’s disease, parkinson-plus syndromes (e.g. progressive supranuclear palsy, multiple system atrophy), normal pressure hydrocephalus, stroke, traumatic brain injury, medication-induced or medical illness -related cognitive impairment, cognitive impairment attributed to psychiatric-illness (other than depression) or substance abuse.
Variables
Data requested from NACC included demographics, item-specific subscores on the UPDRS motor subscale19, Folstein Mini Mental State Exam (MMSE) scores15, estimated age of onset of cognitive decline, and etiological diagnoses. Subjects were divided into three groups based on their clinical phenotypic diagnosis. The AD group carried a solitary primary diagnosis of probable AD based on the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association criteria18. The DLB group consists of those with a solitary, primary diagnosis of DLB based on published consensus criteria1. The “Lewy Body Variant of AD” (LBV) group includes those with dual diagnoses of AD and DLB, with the DLB diagnosis serving as either primary or secondary.
Measures
Primary outcome measures included the UPDRS motor score and the number of extrapyramidal signs present for an individual. Each center contributing data to the NACC had clinicians that were trained in a standardized fashion, using video training provided by the Parkinson’s Study Group, to score the motor items on the UPDRS. The UPDRS motor score was defined as the sum of 27 subitems rated from the neurological examination; any individual missing more than ten subitems had a missing total score and were excluded from the analysis. All 27 subitems scored in the UPDRS motor subscale were then grouped into 9 EPS: Speech/Hypophonia, Masked Facies, Resting Tremor, Action/Postural Tremor, Rigidity, Bradykinesia, Impaired Chair Rise, Impaired Posture/Gait, and Postural Instability. Speech/Hypophonia included the single item grading speech volume and quality. Masked Facies included the single item grading facial expression. Resting Tremor included the 5 subitems grading tremor at rest in the face/lips/chin and 4 extremities. Action/Postural Tremor included intention tremor in both upper extremities. Rigidity included presence and degree of rigidity in the neck and 4 extremities. Bradykinesia included 9 subitems: bilateral finger tapping, hand movements, rapid alternating movements of the hands, leg agility and body bradykinesia. Impaired Chair Rise included the single measure of arising from a chair. Impaired Posture/Gait combined the items posture and gait. Postural Instability included the single item of postural stability. Each of the EPS was graded as absent (score of 0 or 1 out of 4) or present (score of 2–4, inclusive). A score of 1 indicated very mild parkinsonism that could be considered normal for age19; for that reason, a score of 1 did not indicate presence of significant parkinsonism for the main analyses of this study. The total number of signs present was then determined for each subject. However, for two of the UPDRS items, Resting tremor and the gait component of Impaired Posture/Gait, the score of 1 might be considered abnormal regardless of age (e.g. resting tremor “slight and infrequently present”) and suggestive of significant parkinsonism. Therefore, the analyses for predicting a LB phenotype were repeated secondarily in all subjects using this lower cutoff for Resting Tremor and the gait component of Impaired Posture/Gait. Primary variables of interest were diagnostic group and MMSE. Total MMSE scores were stratified into the following categories for analysis: 0–10, 11–15, 16–20, 21–25, 26–30.
Data analyses
Linear regression models were used to assess differences between diagnostic groups in UPDRS total scores, while Poisson regression models were used to assess differences between groups in the number of EPS present. The total UPDRS score was square-rooted prior to analysis to meet the assumptions of the model. All models were adjusted for age and gender. Assumptions of the models were checked numerically and graphically and were met by the data. Secondary analyses compared the prevalence of each EPS across the diagnostic groups using Chi-square tests and the association of EPS with Lewy body phenotype (combined LBV and DLB groups) using logistic regression models adjusted for age, education, gender, and MMSE. Odds ratios and confidence intervals were also computed. Models were first fit individually for each EPS. A forward stepwise logistic regression model was then built to assess the independent associations of the 9 EPS with a Lewy body phenotype (DLB or LBV). This model was adjusted for age, education, gender and MMSE. A joint model including those EPS found to be significantly associated with Lewy body phenotype in the univariate models was also created to compare to the results of the stepwise regression model. A further analysis considered UPDRS total score as a predictor of Lewy body phenotype in a logistic regression model adjusted for age, education, gender and MMSE. All analyses were conducted using SAS, version 9.1 and p-values<0.05 was considered statistically significant.
Results
Sample characteristics
There were a total of 3,429 subjects who met inclusion criteria. However, we excluded 1,368 subjects who either lacked a significant amount of specific UPDRS data (n=1,274) or had excluded clinical diagnoses (n=94) as detailed in the methods section. Therefore, the total number of subjects included in the analyses was 2,061 with 1,826 subjects in the AD group, 130 subjects in the DLB group, and 105 subjects in the LBV group. Table 1 describes the study sample. The DLB group was younger, had a higher MMSE and a higher percentage of males than the AD and LBV groups (p<0.05, corrected for multiple comparisons). This group also had a higher percentage of Caucasians than the AD group (p<0.05, corrected for multiple comparisons). The groups did not differ on level of education. The DLB group had a shorter disease duration than the AD group (p<0.05, corrected for multiple comparisons). The DLB and LBV groups had higher total UPDRS scores and a higher number of EPS present than the AD group.
Table 1.
Sample characteristics expressed as mean (sd) unless otherwise noted.
| Variable | AD (n=1826) | DLB (n=130) | LBV (n=105) | p-value |
|---|---|---|---|---|
| Age | 79.7 (6.9) | 75.3 (6.7) | 78.7 (6.2) | p<0.001 |
| Education | 14.0 (3.8) | 14.7 (3.3) | 14.0 (3.8) | p=0.096 |
| Male (%) | 44 | 79 | 56 | p<0.001 |
| Caucasian (%) | 83 | 91 | 84 | p=0.046 |
| MMSE | 19.6 (6.5) | 21.7 (6.4) | 18.2 (6.7) | p<0.001 |
| Total UPDRS | 8.6 (8.1) | 21.8 (13.2) | 17.1 (11.0) | p<0.001 |
| Number of EPS | 0.9 (1.3) | 3.0 (2.4) | 2.2 (2.0) | p<0.001 |
| Disease Duration (years) | 5.3 (3.5) | 4.3 (2.8) | 5.1 (3.0) | p=0.005 |
Stratification by MMSE
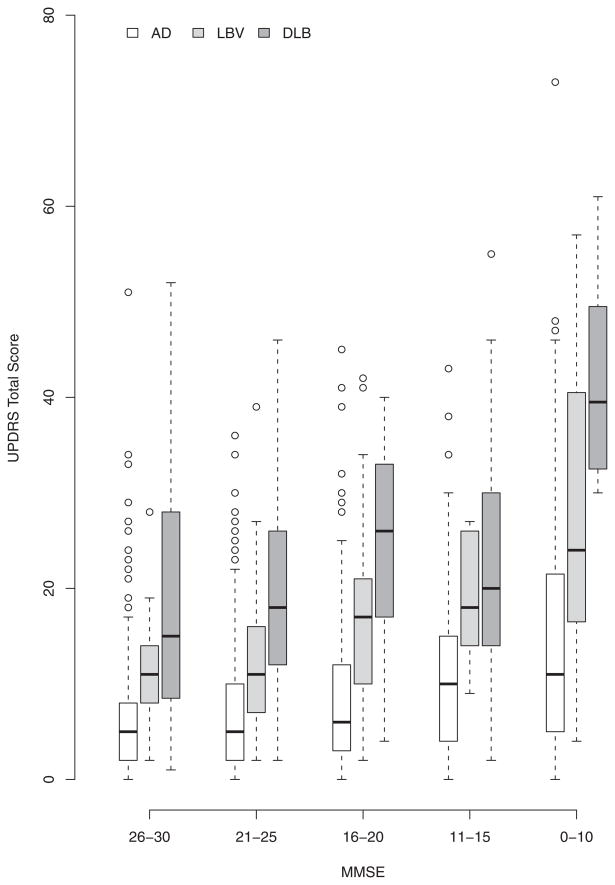
Boxplots of the total UPDRS scores by MMSE strata within each group illustrate a general trend of increasing UPDRS with decreasing MMSE (Figure 1). In the AD group, the median UPDRS in the MMSE 26-30 stratum was 5, while the median UPDRS in the MMSE 0-10 stratum was 11. In the DLB group, the median UPDRS at MMSE 26-30 was 15, as compared to the median UPDRS at MMSE 0-10 of 39.5. The LBV group had a median UPDRS score of 11 at MMSE 26-30 and a median UPDRS of 24 at MMSE 0-10. It is also interesting to note that the AD group had more outliers than did the DLB or LBV groups. In models adjusted for age and gender using the square root of the UPDRS as the outcome, decreasing MMSE remained associated with increasing UPDRS within each group (p<0.001 for each group). As expected, the three groups differed in their total UPDRS scores (p<0.001), after adjusting for age, gender and MMSE, with DLB having the highest scores, followed by LBV and then AD. The total UPDRS for the LBV group with the highest MMSE stratum (26-30) was similar to the UPDRS in the AD group with the lowest MMSE stratum (0-10) (p=0.9). In contrast, the total UPDRS for the DLB group in the highest MMSE stratum (26-30) was significantly higher (p=0.001) than the total UPDRS for the AD group in the lowest MMSE stratum (0-10). Results did not change after adjusting for disease duration.
Figure 1.
Boxplots of total UPDRS scores versus stratified MMSE scores in all diagnostic groups. Circles represent “outliers”, those observations that extended beyond the 75th percentile plus 1.5 times the interquartile range (75th percentile – 25th percentile).
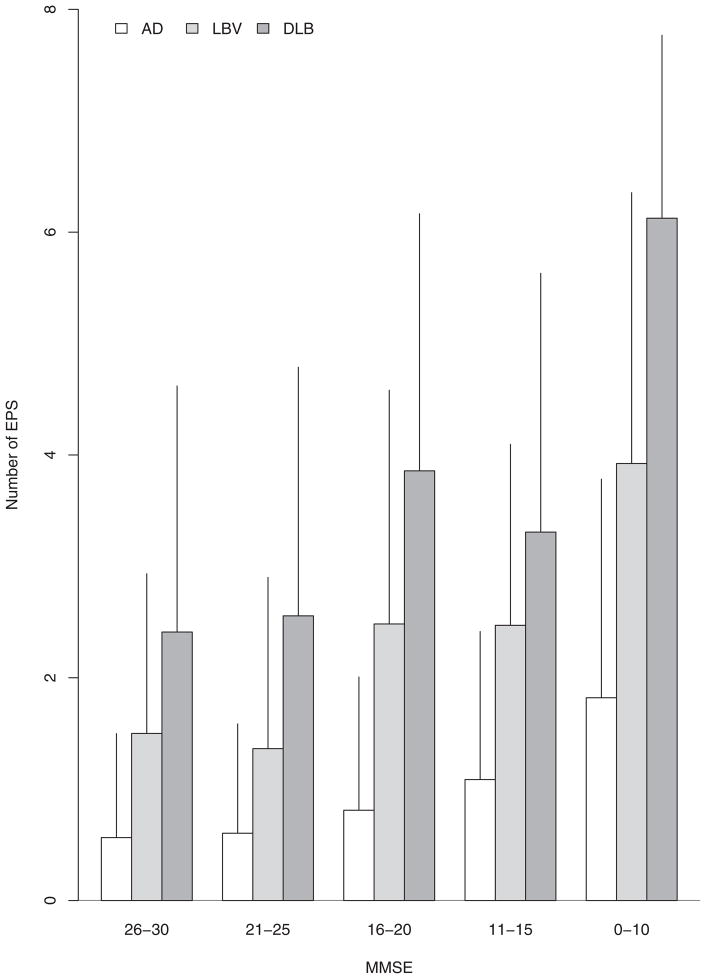
Barplots showing the mean and standard deviation of the total number of EPS (range 0–9) by MMSE strata within each group also illustrate a general trend of increasing number of EPS with decreasing MMSE (Figure 2). As expected, the three groups differed in their total number of EPS (p<0.001), after adjusting for age, gender, and MMSE, with DLB having the highest number of EPS, followed by LBV and then AD. Within each MMSE stratum, there was a similar gradient of number of EPS, with DLB having the highest number, followed by LBV and AD. As with the analysis by total motor UPDRS score, the results did not change after adjusting for disease duration.
Figure 2.
Barplots demonstrating the mean number of EPS versus MMSE strata in all diagnostic groups. Error lines represent one standard deviation.
Analyses by individual EPS
Table 2 gives the prevalence of each extrapyramidal sign by group. For all 9 EPS, the prevalences were highest in the DLB and LBV groups, as compared to the AD group. For 7 of the 9 EPS, the LBV group showed prevalences intermediate to the DLB and AD groups (all EPS except Action/Postural Tremor and Impaired Chair Rise). Further analyses assessed how each EPS was associated with a diagnosis of a Lewy body (LB) disease (DLB or LBV). Table 2 also gives the odds ratios of having a LB diagnosis in relation to the presence or absence of an individual EPS from models adjusted for age, education, and MMSE. As expected, each specific EPS was associated with significantly increased odds of having a LB diagnosis. The odds ratio for Masked Facies (OR=18.6, p<0.001, see tables for confidence intervals) and Hypophonic Speech (OR=14.1, p<0.001) were particularly high. Adjusted odds ratios remain similar for Resting Tremor and Impaired Posture/Gait if the lower cutoffs (scores ≥ 1= abnormal) are used (Table 2 footnote). In the stepwise regression model (Table 3), each of the EPS remained in the model, except for Impaired Chair Rise and Postural Instability. Masked Facies had the highest odds ratio in the stepwise model (OR=6.5, p<0.001) while Action/Postural Tremor had the lowest significant odds ratio (OR=1.9, p=0.01). The joint model showed very similar odds ratios (not shown). The overall prediction accuracy of the stepwise regression model was 83.1%. When this model was repeated without the demographic variables or MMSE score, accuracy fell to 71.4%. Results were similar after adjusting for disease duration. When the total UPDRS score was entered into the model instead of the specific EPS (with the demographic variables and MMSE), the results were very similar, with an overall prediction accuracy of 83.6%. Results of the stepwise regression model with alternate cutoffs for Resting Tremor and Impaired Posture/Gait also remain similar with an overall accuracy of 84.8%(Supplement Table 1). Masked facies still had the highest odds ratio (OR=5.0, p<0.001); Resting Tremor had the lowest (OR=1.8, p= 0.01).
Table 2.
The prevalence of individual extrapyramidal signs in each diagnostic group, and odds ratios for a Lewy body diagnosis (DLB+LBV) from univariate models (adjusted for age, education, gender and MMSE).
| Within Group Prevalence (%)* | Unadjusted OR of LB diagnosis* [95% CI] | Adjusted OR of LB diagnosis* [95% CI] | ||||
|---|---|---|---|---|---|---|
| AD | DLB | LBV | ||||
| Masked Facies | 4.2 | 48.5 | 24.8 | 14.0 [9.9–19.9] | 18.6 [12.2–28.3] | |
| Speech/Hypophonia | 3.4 | 30.8 | 11.4 | 8.1 [5.4–12.0] | 14.1 [8.4–23.4] | |
| Rigidity | 9.8 | 44.6 | 37.1 | 6.5 [4.8–8.7] | 7.0 [5.1–9.8] | |
| Impaired Posture/Gait | 14.2 | 43.1 | 31.4 | 3.7 [2.7–4.9] | 6.4 [4.4–9.1] | |
| Bradykinesia | 19.3 | 55.4 | 46.7 | 4.4 [3.3–5.8] | 5.9 [4.3–8.1] | |
| Impaired Chair Rise | 15.4 | 28.9 | 28.7 | 2.2 [1.6–3.0] | 4.5 [3.1–6.5] | |
| Resting Tremor | 3.2 | 13.9 | 7.6 | 3.7 [2.3–6.0] | 3.9 [2.4–6.6] | |
| Postural Instability | 12.5 | 26.2 | 20.6 | 2.2 [1.6–3.1] | 3.8 [2.6–5.6] | |
| Action/Postural Tremor | 6.2 | 12.3 | 18.1 | 2.6 [1.7–3.9] | 2.5 [1.6–3.8] | |
Using a cutoff of ≥1 for Resting Tremor resulted in an adjusted odds ratio of 3.0 [2.1–4.2], with a prevalence of 11.9% in AD subjects, 30.8% in DLB and 23.8% in LBV. Using ≥2 for posture and ≥1 for the gait component in Impaired Posture/Gait, yielded an OR of 7.7 [5.4–11.0] with prevalences of 42.1% in AD, 82.3% in DLB, and 71.4% in LBV
All p-values <0.0001
Table 3.
Results of the stepwise logistic regression model. This model produced an 83.1% concordance rate in discriminating AD from LB disease (DLB+LBV).
| Estimate | SE | p-value | OR | 95% CI | |
|---|---|---|---|---|---|
| Masked Facies | 1.87 | 0.28 | <0.001 | 6.5 | 3.8–11.1 |
| Rigidity | 1.10 | 0.22 | <0.001 | 3.0 | 2.0–4.6 |
| Resting Tremor | 0.97 | 0.35 | 0.006 | 2.6 | 1.3–5.2 |
| Impaired Posture/Gait | 0.91 | 0.25 | <0.001 | 2.5 | 1.5–4.0 |
| Speech/Hypophonia | 0.83 | 0.37 | 0.025 | 2.3 | 1.1–4.7 |
| Bradykinesia | 0.78 | 0.21 | <0.001 | 2.2 | 1.4–3.3 |
| Action/Postural Tremor | 0.65 | 0.26 | 0.01 | 1.9 | 1.1–3.2 |
| Gender: Male | 0.89 | 0.19 | <0.001 | 2.4 | 1.7–3.5 |
| MMSE | 0.09 | 0.02 | <0.001 | 1.1 | 1.06–1.13 |
| Education | −0.03 | 0.02 | 0.17 | 0.97 | 0.92–1.01 |
| Age | −0.07 | 0.01 | <0.001 | 0.93 | 0.90–0.95 |
Intercept= −4.00 ± 0.51
Discussion
The presence of motor parkinsonism, as measured by the UPDRS, is not specific to any single neurodegenerative disorder; however, the severity of motor parkinsonism and specific EPS may indicate a higher likelihood of a clinical DLB diagnosis. In this study, patients with DLB had the most severe parkinsonism and number of EPS, in comparison to those with LBV and AD. They were also generally younger, were more often male and had slightly higher MMSE scores than those with AD. Of the 3 groups studied, AD subjects had the lowest UPDRS scores and the fewest discrete signs of motor parkinsonism. However, AD subjects had some degree of measurable parkinsonism, which worsened with the cognitive severity of the disease. Those subjects with late-stage AD (MMSE 0-10) had a similar degree of parkinsonism to those with early-stage LBV (MMSE 26-30), but had significantly less parkinsonism than those subjects with early-stage DLB (MMSE 26-30). This finding, therefore, might assist the clinician with the diagnosis of DLB, particularly early in the course of the disease.
After describing the degree and number of EPS, our next goal was to identify specific EPS that might help the clinician differentiate AD and LB disease in the clinical setting. Logistic regression models consistently demonstrate that all 9 EPS differentiate DLB from AD, regardless of age, gender, education or MMSE. Furthermore, 7 of the 9 EPS were each independently associated with LB disease, in descending order: Masked Facies, Rigidity, Resting Tremor, Impaired Posture/Gait, Speech/Hypophonia, Bradykinesia and Action/Postural Tremor. The final forward stepwise model did not include Impaired Chair Rise or Postural Instability after accounting for the other 7 EPS. These two signs may be less specific for DLB, as other etiologies common in the geriatric population may also produce these signs, e.g. arthropathy of the knees and/or lumbar spine, coexisting cerebrovascular disease, vestibular dysfunction or peripheral neuropathy with sensory ataxia. These findings were similar to a principal components analysis done by Ballard, et al20, which demonstrate, in a different population (N=42 DLB, 30 AD), that rest tremor, action tremor, bradykinesia, facial expression and rigidity subscales of the UPDRS were helpful in differentiating DLB from AD. Another study by Galasko et al,21 also showed that masked facies, rigidity, parkinsonian gait, and bradykinesia discriminated between pathology-verified AD and LB disease in a dementia cohort. Our model was based on a much larger sample and included independent associations of MMSE, gender and age, such that male gender, higher MMSE scores, and younger age were associated with a diagnosis of LB disease. It should be noted that including demographic variables and dementia severity (MMSE) to the model markedly improved discrimination between groups, beyond that achieved by considering EPS alone. The DLB group had the highest presence of EPS and the most severe motor parkinsonism overall, despite having shorter estimated disease duration.
There are several limitations of the study. The model assumes that the individual EPS are independent, though we recognize that they likely are not truly independent. One aspect of this issue is that two parkinsonian signs (e.g. masked facies, bradykinesia) may co-occur due to the same underlying pathophysiology. Also, the clinical examiner may be influenced by the diagnostic impression or the presence/absence of another sign in determining if an individual EPS is present. There is some circular logic to the use of parkinsonism in the diagnostic criteria defining the groups studied, then studying the degree of parkinsonism in those groups. As a result, interpreting results using the total UPDRS score is significantly limited. One remedy for this limitation would be use of objective quantitative measures for parkinsonian signs such as rigidity22, tremor23, and bradykinesia24. These measures deserve further study and development in dementia cohorts. Another limitation of using the total UPDRS motor score is that the UPDRS was developed for measuring symptom severity in Parkinson’s disease, not as a diagnostic tool in dementia patients. Some academic dementia clinics have shown both utility and good inter-rater reliability25–27 for the UPDRS motor subscale and similar scales in dementia cohorts; however, there are no published inter-rater reliability studies for the UPDRS as it is used in the NACC database.
Another study limitation is that the cohort studied is a clinical referral population, not a community- or population-based cohort, limiting the generalizability of the study results. The use of the term LBV remains controversial and further research into this entity is needed. Our use of the term LBV is meant to capture those subjects who meet criteria for both AD and DLB and have increased likelihood to carry both pathologies. Most cases of DLB are mixed (>84%), with relatively few cases of pure DLB without AD pathology28–30. The degree of overlapping AD pathology coexistent with LB pathology may mask core symptoms of LB disease28. In the LBV cases in this study, both pathologies may be present and contribute to the clinical picture, such that the subject meets criteria for both AD and DLB. There is, however, a weakness in the DLB critieria1, which excludes the presence of other coexisting illnesses, such as AD, which may contribute to the clinical phenotype. If the DLB criteria are strictly interpreted, these mixed cases, which would otherwise meet criteria for DLB, would be classified solely as AD. This reflects the ongoing challenge of accurately identifying mixed pathologies ante-mortem. The utility of clinically differentiating LBV from pure DLB is not proven, but these overlap cases may have more severe cholinergic deficits31–33. Lastly, this study has not yet been validated by neuropathology in this cohort; future neuropathologic studies are planned once larger samples are available. Prior reports from other Alzheimer’s Disease Centers in the United States have found low sensitivity of the consensus DLB criteria in pathologically confirmed DLB cases3,4,10,13. Higher sensitivity of the criteria to LB disease has been reported by the Newcastle group2, which utilized a geropsychiatry cohort, in addition to having a tertiary DLB referral center.
The strengths of this study include sample size, statistical power, statistical methods and the use of the NACC database. NACC obtains data from nationwide tertiary-referral centers that utilize widely used and validated clinical measures, such as the UPDRS and diagnostic criteria. The UPDRS data allowed for identification of a profile of motor parkinsonism and demographics associated with Lewy body disease. The very large sample size and stratification by MMSE, a commonly used clinical tool that approximates dementia stage and severity, makes this study a unique addition to the literature. Independent replication and validation of these models and results may allow the more refined use of EPS to improve diagnostic accuracy in the future.
Differentiating AD and DLB remains a clinical challenge. The differentiation of these disorders is important. Currently, the major treatment difference between DLB and AD is to avoid typical neuroleptic agents and other phenothiazines1. Increased sensitivity to the newer antipsychotic drugs may also been seen in DLB34. Once specific therapies emerge to treat the alpha-synucleinopathies, accurate early detection of DLB will become much more important. In this sample, the severity of parkinsonism, in addition to MMSE and demographic data, can help differentiate DLB from AD. Group differences, such as those found in this study, are not always useful at the individual level. The sensitivity and specificity of these EPS, as compared to the neuropathological diagnostic criteria for DLB1, needs to be determined before we can be confident of their clinical utility. If verfied by future autopsy studies, however, the presence of specific signs of parkinsonism such as masked facies and hypophonic speech, may help clinicians more efficiently diagnose DLB in the clinical setting.
Supplementary Material
Acknowledgments
This study was funded by the NIA Director’s Reserve Supplement and the following NIA grants: U01 AG016976, P30 AG10129 and P30 AG01961. There are no industry-sponsors of this study. Many thanks to Dr. Walter Kukull, Leslie Phillips, the NACC publications committee, and the NACC staff and faculty. for their assistance in obtaining the data and reviewing this manuscript.
Abbreviations
- AD
Alzheimer’s disease
- DLB
dementia with Lewy bodies
- EP
extrapyramidal
- EPS
extrapyramidal sign(s)
- LB
Lewy body
- LBV
Lewy body variant of AD
- MMSE
Mini Mental Status Exam
- NACC
National Alzheimer’s Coordinating Center
- OR
odds ratios
- UPDRS
United Parkinson’s Disease Rating Scale
Footnotes
Conflicts of Interest: None of the authors had any relevant disclosures to declare
References
- 1.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies. Neurology. 2005 Dec 27;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 2.McKeith IG, Ballard CG, Perry RH, et al. Prospective validation of Consensus criteria for the diagnosis of dementia with Lewy bodies. Neurology. 2000 Mar 14;54(5):1050–1058. doi: 10.1212/wnl.54.5.1050. [DOI] [PubMed] [Google Scholar]
- 3.Lopez OL, Becker JT, Kaufer DI, et al. Research Evaluation and Prospective Diagnosis of Dementia With Lewy Bodies. Arch Neurol. 2002 Jan 1;59(1):43–46. doi: 10.1001/archneur.59.1.43. [DOI] [PubMed] [Google Scholar]
- 4.Nelson PT, Jicha GA, Kryscio RJ, et al. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol. 2010 Mar;257(3):359–366. doi: 10.1007/s00415-009-5324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKeith IG, Rowan E, Askew K, et al. More severe functional impairment in dementia with lewy bodies than Alzheimer disease is related to extrapyramidal motor dysfunction. Am J Geriatr Psychiatry. 2006 Jul;14(7):582–588. doi: 10.1097/01.JGP.0000216177.08010.f4. [DOI] [PubMed] [Google Scholar]
- 6.Olichney JM, Galasko D, Salmon DP, et al. Cognitive decline is faster in Lewy body variant than in Alzheimer’s disease. Neurology. 1998 Aug;51(2):351–357. doi: 10.1212/wnl.51.2.351. [DOI] [PubMed] [Google Scholar]
- 7.Samuel W, Alford M, Hofstetter CR, Hansen L. Dementia with Lewy bodies versus pure Alzheimer disease: differences in cognition, neuropathology, cholinergic dysfunction, and synapse density. J Neuropathol Exp Neurol. 1997 May;56(5):499–508. doi: 10.1097/00005072-199705000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Clerici F, Ratti PL, Pomati S, Maggiore L, Elia A, Mariani C. Cholinergic balance in dementia with Lewy bodies: reversible worsening of Parkinsonism at rivastigmine dosage modulation. Neurol Sci. 2007 Oct;28(5):282–284. doi: 10.1007/s10072-007-0837-6. [DOI] [PubMed] [Google Scholar]
- 9.Richard IH, Justus AW, Greig NH, Marshall F, Kurlan R. Worsening of motor function and mood in a patient with Parkinson’s disease after pharmacologic challenge with oral rivastigmine. Clin Neuropharmacol. 2002 Nov-Dec;25(6):296–299. doi: 10.1097/00002826-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Mega MS, Masterman DL, Benson DF, et al. Dementia with Lewy bodies: reliability and validity of clinical and pathologic criteria. Neurology. 1996 Dec;47(6):1403–1409. doi: 10.1212/wnl.47.6.1403. [DOI] [PubMed] [Google Scholar]
- 11.Ferman TJ, Smith GE, Boeve BF, et al. DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology. 2004 Jan 27;62(2):181–187. doi: 10.1212/wnl.62.2.181. [DOI] [PubMed] [Google Scholar]
- 12.Walker MP. The Clinician Assessment of Fluctuation and the One Day Fluctuation Assessment Scale: Two methods to assess fluctuating confusion in dementia. The British Journal of Psychiatry. 2000;177(3):252–256. doi: 10.1192/bjp.177.3.252. [DOI] [PubMed] [Google Scholar]
- 13.Fujishiro H, Ferman TJ, Boeve BF, et al. Validation of the neuropathologic criteria of the third consortium for dementia with Lewy bodies for prospectively diagnosed cases. J Neuropathol Exp Neurol. 2008 Jul;67(7):649–656. doi: 10.1097/NEN.0b013e31817d7a1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Stern Y, Chun MR, Jacobs DM, Yau P, Goldman JE. Pathological correlates of extrapyramidal signs in Alzheimer’s disease. Ann Neurol. 1997 Mar;41(3):368–374. doi: 10.1002/ana.410410312. [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer’s Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007 Jul-Sep;21(3):249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 17.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004 Sep;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 18.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984 Jul;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 19.Fahn S, Elton R. UPDRS Development Committee. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden C, Calne DB, Goldstein M, editors. Recent Developments in Parkinson’s Disease. Vol. 2. Florham Park, NH: Macmillan; 1987. pp. 153–163.pp. 293–304. [Google Scholar]
- 20.Ballard C, McKeith I, Burn D, et al. The UPDRS scale as a means of identifying extrapyramidal signs in patients suffering from dementia with Lewy bodies. Acta Neurol Scand. 1997 Dec;96(6):366–371. doi: 10.1111/j.1600-0404.1997.tb00299.x. [DOI] [PubMed] [Google Scholar]
- 21.Galasko D, Katzman R, Salmon DP, Hansen L. Clinical and Neuropathological Findings in Lewy Body Dementias. Brain Cogn. 1996;31(2):166–175. doi: 10.1006/brcg.1996.0040. [DOI] [PubMed] [Google Scholar]
- 22.Mak MK, Wong EC, Hui-Chan CW. Quantitative measurement of trunk rigidity in parkinsonian patients. J Neurol. 2007 Feb;254(2):202–209. doi: 10.1007/s00415-006-0327-4. [DOI] [PubMed] [Google Scholar]
- 23.Hoff JI, Wagemans EA, van Hilten BJ. Ambulatory Objective Assessment of Tremor in Parkinson’s Disease. Clin Neuropharmacol. 2001;24(5):280–283. doi: 10.1097/00002826-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Dunnewold RJ, Jacobi CE, van Hilten JJ. Quantitative assessment of bradykinesia in patients with Parkinson’s disease. J Neurosci Methods. 1997 Jun 6;74(1):107–112. doi: 10.1016/s0165-0270(97)02254-1. [DOI] [PubMed] [Google Scholar]
- 25.Bennett DA, Shannon KM, Beckett LA, Wilson RS. Dimensionality of parkinsonian signs in aging and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 1999 Apr;54(4):M191–196. doi: 10.1093/gerona/54.4.m191. [DOI] [PubMed] [Google Scholar]
- 26.Richards M, Marder K, Bell K, Dooneief G, Mayeux R, Stern Y. Interrater Reliability of Extrapyramidal Signs in a Group Assessed for Dementia. Arch Neurol. 1991 Nov 1;48(11):1147–1149. doi: 10.1001/archneur.1991.00530230055021. [DOI] [PubMed] [Google Scholar]
- 27.Richards M, Bell K, Dooneief G, et al. Patterns of neuropsychological performance in Alzheimer’s disease patients with and without extrapyramidal signs. Neurology. 1993 Sep 1;43(9):1708. doi: 10.1212/wnl.43.9.1708. [DOI] [PubMed] [Google Scholar]
- 28.Merdes AR, Hansen LA, Jeste DV, et al. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology. 2003 May 27;60(10):1586–1590. doi: 10.1212/01.wnl.0000065889.42856.f2. [DOI] [PubMed] [Google Scholar]
- 29.Hohl U, Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J. Diagnostic accuracy of dementia with Lewy bodies. Arch Neurol. 2000 Mar;57(3):347–351. doi: 10.1001/archneur.57.3.347. [DOI] [PubMed] [Google Scholar]
- 30.Kosaka K. Diffuse Lewy body disease in Japan. J Neurol. 1990 Jun;237(3):197–204. doi: 10.1007/BF00314594. [DOI] [PubMed] [Google Scholar]
- 31.Fujishiro H, Umegaki H, Isojima D, Akatsu H, Iguchi A, Kosaka K. Depletion of cholinergic neurons in the nucleus of the medial septum and the vertical limb of the diagonal band in dementia with Lewy bodies. Acta Neuropathol. 2006 Feb;111(2):109–114. doi: 10.1007/s00401-005-0004-1. [DOI] [PubMed] [Google Scholar]
- 32.Bohnen NI, Kaufer DI, Ivanco LS, et al. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch Neurol. 2003 Dec;60(12):1745–1748. doi: 10.1001/archneur.60.12.1745. [DOI] [PubMed] [Google Scholar]
- 33.Langlais PJ, Thal L, Hansen L, Galasko D, Alford M, Masliah E. Neurotransmitters in basal ganglia and cortex of Alzheimer’s disease with and without Lewy bodies. Neurology. 1993 Oct;43(10):1927–1934. doi: 10.1212/wnl.43.10.1927. [DOI] [PubMed] [Google Scholar]
- 34.Friedman JH, Fernandez HH. Atypical antipsychotics in Parkinson-sensitive populations. J Geriatr Psychiatry Neurol. 2002 Fall;15(3):156–170. doi: 10.1177/089198870201500307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.