Abstract
Background and Objectives. Atypical lipomas are uncommon, slow-growing benign tumors. While surgery has been the primary treatment modality, we have managed some patients with radiation (RT) as a component of the treatment and have reported their outcomes in this study. Methods. A retrospective review of all cases of extremity and trunk atypical lipomas in The Sarcoma Database at the study institution was conducted. Results. Thirteen patients were identified. All patients underwent surgical resection at initial presentation and received pre- or postoperative radiation for subtotal resection (n = 2), local recurrence (n = 8), or progressive disease (n = 3). The median total radiation dose was 50 Gy. Median followup was 65.1 months. All patients treated with RT remained free of disease at the last followup. No grade 3 or higher late toxicity from radiation was observed. No cases of tumor dedifferentiation occurred. Conclusion. For recurrent or residual atypical lipomas, a combination of reexcision and RT can provide long-term local control with acceptable morbidity. For recurrent tumors, pre-op RT of 50 Gy appears to be an effective and well-tolerated management approach.
1. Introduction
The term atypical lipoma was first introduced in 1974 [1] to describe lobulated, well-circumscribed, large lesions that are histologically characterized by mature adipocytes of varying size and scattered atypical stromal cells with hyperchromatic nuclei (Figure 1) [2, 3]. Atypical lipomas are slow-growing tumors that typically affect the extremities and trunk. In the absence of a dedifferentiated component, atypical lipomas do not metastasize and pattern of failure is predominantly local [3–11]. The primary treatment modality has been surgical resection, with reexcision for recurrent lesions.
Figure 1.
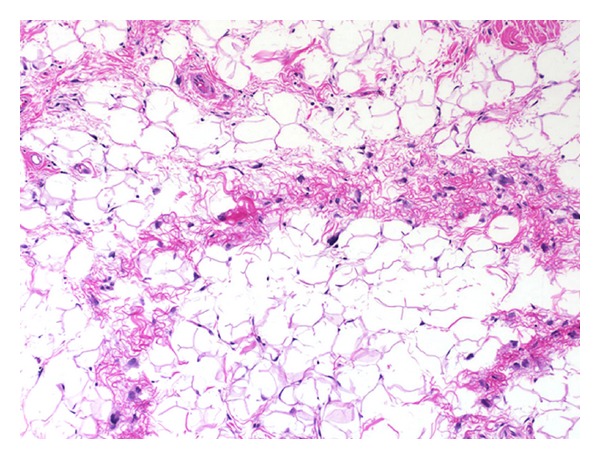
Histologic presentation of atypical lipoma. Atypical lipoma composed of mature fat and fibrous tissue with scattered, enlarged, hyperchromatic cells.
The role and utility of preoperative or adjuvant radiation in management of these lesions is not well known. We have managed some patients with recurrent or subtotally resected tumors with radiation as a component of the treatment. In this study, we review the outcomes of thirteen patients with atypical lipoma treated with radiation therapy (RT) at the Massachusetts General Hospital during the years of 1995–2010.
2. Methods
A retrospective review of all cases of atypical lipoma treated with radiation at the Massachusetts General Hospital was conducted. Patients were excluded if RT was completed after January 2010. Thirteen consecutively treated patients were identified, initially diagnosed between 1987 and 2007. Median age at diagnosis was 54 (range: 36–76 years).
With the exception of one chest wall lesion, all lesions were located in the upper (n = 4) or lower (n = 8) extremity. Of the lower extremity lesions, seven were in the thigh and one was in the calf. Of the upper extremity lesions, two were in the forearm, one in the deltoid, and one in the proximal arm.
Details of each patient and treatment are shown in Table 1. Tumors were diagnosed based on reported histologic features after pathology review at Massachusetts General Hospital. Toxicity was graded based on Common Terminology Criteria for Adverse Events v4.0 (CTCAE).
Table 1.
Individual patient and treatment details.
| Sex | Age at diagnosis (Y) |
Site | Initial management | Size of initial lesion | Time to LR/treatment of PD (M) |
Treatment for progression/LR | Size of recurrent lesion | Total RT Dose (Gy)/fractions | Modality | FU (M) |
Toxicity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | 47 | Thigh | GTR | 6.0 × 6.0 × 6.0 | 20 | Preop RT + GTR | 5.0 × 3.1 × 0.5, 2.0 × 1.0 × 0.5 |
50/25 | Ph | 35.4 | |
| F | 76 | Thigh | GTR | 4.0 × 3.0 × 1.5 | 20.2 | Preop RT + GTR | 10.0 × 8.7 × 2.7 | 50/25 | Ph | 47.2 | Post-op wound infection requiring IV abx, Grade 1 edema |
| M | 65 | Forearm | GTR | 15.0 × 5.0 × 5.0 | 36.7 | Preop RT +GTR | 8 × 7 × 3 | 50/25 | Ph | 7.5 | |
| F | 73 | Thigh | GTR | 6.0 × 9.0 | 124.2 | GTR + postop RT | 12.5 × 8.3 × 8.0 | 66/33 | Ph | 79.8 | Grade 1 edema |
| F | 45 | Thigh | GTR | 10.5 × 6.5 × 2 | 133.8 | GTR + postop RT | 14.0 × 8.0 × 4.1 | 50/25 | Ph | 147.2 | |
| F | 39 | Thigh | Resection, followed by LR at 4 y, managed with GTR | Unknown | 16.3 | Preop RT + GTR | 7.0 × 6.0 × 35 | 50/25 | Ph | 68.1 | |
| M | 46 | Thigh | Resection, followed by LR at 11 y, treated with re-excision | Unknown | 124.5 | Preop RT + GTR | 19 × 10.5 × 4.0 | 50/25 | Ph | 3.7 | |
| F | 55 | Forearm | Resection, followed by LR at 2 y, treated with re-excision; followed by LR at 3 y, treated with re-excision | Unknown | 87.9 | GTR + brachytherapy | 8.5 × 4.5 × 1.4 | 60/1 | Ir-192 | 148.9 | Grade 1 telangiectasia |
| M | 41 | Deltoid | STR + chemotherapy + re-excision | Unknown | 27.4 | Preop RT + GTR | 11 × 9 × 14.5 | 50/25 | Ph | 21.3 | |
| F | 58 | Thigh | STR | 35.0 × 14.0 × 7.0 | 58.1 | GTR + postop RT | Unknown | 61.2/34 | Ph | 65.1 | |
| M | 66 | Thigh | STR | 20.0 × 18.0 × 3.0 | 46.3 | RT alone | 18.2 × 6.5 × 3.4 | 70/35 | Ph + Pr | 48.7 | |
| F | 36 | Thigh | STR + postop RT | 21.0 × 16.0 × 2.8 | N/A | N/A | N/A | 60/30 | Ph | 196.0 | Grade 2 sciatic neuropathy |
F indicates female, M: male; Y: years; GTR: gross total resection; STR: subtotal resection; RT: radiation; N/A: not applicable; LR: local recurrence; PD: progressive disease; M: months; Gy: Gray; Ph: photons; Pr: protons.
Gross total resection (GTR) was defined as tumor resected with negative margins. Subtotal resection (STR) was defined as gross residual disease. Local recurrence was defined as regrowth of tumor after a gross total resection. Progressive disease was defined as growth of residual tumor after STR.
All the patients underwent surgical resection at initial presentation, either GTR (n = 6), STR (n = 4), or extent of resection unknown (n = 3).
Of patients with GTR, local recurrence was identified at a mean of 58.5 months (range, 20.0–133.8 months) following resection. The locally recurrent tumor was treated with either preoperative RT (50 Gy in 2 Gy fractions) and GTR (n = 3), or resection followed by postoperative RT (50–66 Gy in 2 Gy fractions) (n = 3).
Of the group of four patients with STR, two were monitored clinically and treated after evidence of disease progression, and the remaining two were treated adjuvantly with radiation for the following reasons. The first adjuvantly treated patient had atypical lipoma of the chest wall, and underwent tumor excision at an outside institution with gross residual disease. He presented with limb paresthesias prior to resection. He was recommended radiation followed by reexcision over observation due to his symptoms, and also because it was felt that a local recurrence would be difficult to resect given the tumor location. He received 50 Gy in 2 Gy fractions, followed by gross total excision of residual tumor. A second adjuvantly treated patient had subtotal excision of tumor that was overlying the sciatic nerve. Adjuvant radiation to 60 Gy in 30 fractions was administered due to concern of residual disease left within the region of the sciatic nerve. The remaining two patients, who were monitored clinically after STR, ultimately required RT with (n = 1) or without excision (n = 1) for progressive disease. The first patient in this group had a subtotal excision of atypical lipoma arising from the thigh, with postoperative scans revealing slow growth of residual tumor. The patient opted for reresection, 58.1 months after initial resection. This was followed by adjuvant radiation to 61.2 Gy in 34 fractions. The other patient underwent initial subtotal resection of an anterior compartment tumor of the leg, and surgery was not recommended due to potential functional morbidity (foot drop). He ultimately received radiation to 70 Gy in 2 Gy fractions to residual tumor, measuring 18.2 × 6.5 × 3.4 cm in size. This was delivered with a combination of photons and protons, 46.3 months after initial resection, due to slow increase in size. Posttreatment scans showed no further tumor growth.
Three patients had surgery with extent of initial resection unknown. One patient received excision, chemotherapy, and reexcision (all delivered in Greece; details of surgical extent and chemotherapy are unknown), followed by local recurrence after 27.4 months, which was treated with preoperative RT and excision. The second patient underwent two excisions in a span of 12 years, followed by local recurrence 10.3 years after last resection, also treated with preoperative RT and excision. The third patient underwent three excisions within a span of five years, followed by local recurrence 5 years after last resection, and was managed with excision and brachytherapy with RT delivered five days postoperatively with low-dose rate Ir-192 brachytherapy to a dose of 60 Gy prescribed to 5 mm from the plane of the implant.
3. Results
Median followup from time of last radiation treatment was 65.1 months (range, 3.7–196.0 months; average 73.1 months). Local tumor control was achieved in all 13 patients treated with radiation. There were no cases of metastatic disease or tumor dedifferentiation on followup. Summary of treatment is shown in Table 2.
Table 2.
Treatment and follow-up summary.
| Sex | Age at diagnosis (Y) |
Site | Initial management | Size of initial lesion | Time to LR/treatment of PD (M) |
Treatment for progression/LR |
|---|---|---|---|---|---|---|
| F | 47 | Thigh | GTR | 6.0 × 6.0 × 6.0 | 20 | Reop RT + GTR |
| F | 76 | Thigh | GTR | 4.0 × 3.0 × 1.5 | 20.2 | Reop RT + GTR |
| M | 65 | Forearm | GTR | 15.0 × 5.0 × 5.0 | 36.7 | Reop RT + GTR |
| F | 73 | Thigh | GTR | 6.0 × 9.0 | 124.2 | GTR + postop RT |
| F | 45 | Thigh | GTR | 10.5 × 6.5 × 2 | 133.8 | GTR + postop RT |
RT indicates radiation; GTR: gross total resection, STR: subtotal resection, FU: follow-up, M: months.
One patient, who received preoperative RT (50 Gy in 2 Gy fractions), developed a postoperative wound infection, requiring hospitalization and administration of IV antibiotics two months after surgery. She recovered fully.
No cases of grade 3 or higher late toxicity from radiation were observed. There were two patients who developed grade 1 lower extremity edema in the treated limb. This includes the patient with grade 3 infection, as detailed above, and a patient who received resection and postoperative RT after initial gross total resection. One patient developed grade 1 telangiectasias; this patient had received brachytherapy to 60 Gy. Another patient, who was treated at initial presentation with subtotal excision followed by adjuvant RT (60 Gy in 2 Gy fractions), developed grade 2 sciatic neuropathy, requiring narcotics for pain control. Of note, she had tumor overlying the sciatic nerve at presentation.
4. Discussion
Atypical lipomas are well-differentiated, slow-growing tumors that typically occur in the trunk and extremities and have a long natural history. Although surgery is the primary treatment modality, the likelihood of local recurrence is considered to be significant. It is difficult to estimate the true rates of local failure from published series due to the small number of patients and heterogeneity in tumor classification, but reported rates range from 8–52% (Table 3) [1, 3, 5–10, 12, 14, 15]. Rates of tumor dedifferentiation range from 0 to 13% in published reports [3–6, 8–10, 13–15]. No cases of tumor dedifferentiation occurred in this present series.
Table 3.
Summary of outcomes reported for atypical lipomas in select series.
| Author | Year | No. of patients | Mean FU (M) |
LR | Dedifferentiation |
|---|---|---|---|---|---|
| n (%) | n (%) | ||||
| Evans et al. [12] | 1979 | 22 | 9 (41%) | 0 | |
| Azumi et al. [7] | 1987 | 48 | 84 | 7 (15) | 0 |
| Weiss and Rao [8] | 1992 | 46 | 108 | 20 (46) | 3 (7) |
| Lucas et al. [9] | 1994 | 32 | 112 | 15 (47) | 6 (19) |
| Rozental et al. [13] | 2002 | 31 | 84 | 16 (52) | 2 (6) |
| Kooby et al. [3] | 2004 | 91 | 47 | 20 (22) | 3 (3) |
| Bassett et al. [14] | 2005 | 51 | 52 | 14 (27) | 1 (2) |
| Sommerville et al. [15] | 2005 | 61 | 50 | 5 (8) | 0 |
| Evans [5] | 2007 | 11a | >120 | 1 (9) | 0 |
| Serpell and Chen [10] | 2007 | 6b | 18 | 3 (50) | 1 (17) |
| Billing et al. [4] | 2008 | 38 | 90 | 4 (10) | 0 |
| Mavrogenis et al. [6] | 2011 | 67 | 81 | 5 (11) | 1 (2) |
FU indicates follow-up, M: months, LR: local recurrence.
aExtremity lipomas only, batypical lipomas only.
Subclassification of atypical lipomas has been explored by several series in an effort to identify factors associated with local recurrence; however, due to small sample sizes, results have been inconclusive. One report by Kooby et al. [3] reviewed 91 lipomatous tumors and identified sclerosing histology to be associated with higher likelihood of local failure. A positive margin was also identified to be associated with increased failures, suggesting these subsets of patients should receive reexcision and consideration of adjuvant treatment.
Standard management of local recurrence is reexcision. However, RT may have a role in providing long-term local control, particularly in areas where wide margins are challenging to achieve. To our knowledge, to date, there has not been any literature focusing on the use of RT in management of recurrent or incompletely resected atypical lipoma.
In this paper, eleven patients were treated for recurrent disease. All patients initially received surgical resection as primary treatment, with average time to recurrence of 63.2 months. Of this group, ten received a combination of RT and surgery and one received RT alone. At median followup of 48.7 months, all patients remained free of disease, demonstrating that long-term control can be achieved with the addition of RT.
Two patients received adjuvant RT at initial presentation due to gross residual disease. At last followup (of 196.0 and 81.0 months, resp.), the patients remained free of recurrent disease, suggesting that long-term local control can be achieved with RT even in the setting of residual tumor.
The potential long-term morbidity of RT and surgery is important to consider when devising a management strategy. One patient, who received 60 Gy of adjuvant radiation following subtotal resection, developed late grade 2 sciatic neuropathy. Preoperative RT is typically delivered to a lower dose, typically 50 Gy, and carries less risk of late fibrosis [16] with long-term control equivalent to postoperative RT. Of patients with recurrent atypical lipomas, 6 received preoperative RT followed by gross tumor resection, and 3 underwent surgery followed by adjuvant RT. No grade 3 or higher late toxicity from radiation was observed, and all patients had control of their tumors.
In conclusion, atypical lipomas are slow-growing tumors with a propensity to recur locally; thus, long-term followup is recommended. RT can provide durable local control for patient and is recommended for patients at especially high risk of recurrence, due to multiply recurrent disease or residual gross tumor. For recurrent tumors, our results suggest a combination of reexcision and RT can provide long-term local control with acceptable morbidity. In the setting of gross residual disease, adjuvant RT can be considered to prevent local progression.
Authors' Contribution
F. J. Hornicek and T. F. DeLaney are co-senior authors in this paper.
References
- 1.Kindblom LG, Angervall L, Stener B, Wickbom I. Intermuscular and intramuscular lipomas and hibernomas: a clinical, roentgenologic, histologic, and prognostic study of 46 cases. Cancer. 1974;33(3):754–762. doi: 10.1002/1097-0142(197403)33:3<754::aid-cncr2820330322>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Mentzel T, Fletcher CD. Lipomatous tumours of soft tissues: an update. Virchows Archiv. 1995;427(4):353–363. doi: 10.1007/BF00199383. [DOI] [PubMed] [Google Scholar]
- 3.Kooby DA, Antonescu CR, Brennan MF, Singer S. Atypical lipomatous tumor/well-differentiated liposarcoma of the extremity and trunk wall: importance of histological subtype with treatment recommendations. Annals of Surgical Oncology. 2004;11(1):78–84. doi: 10.1007/BF02524350. [DOI] [PubMed] [Google Scholar]
- 4.Billing V, Mertens F, Domanski HA, Rydholm A. Deep-seated ordinary and atypical lipomas: histopathology, cytogenetics, clinical features, and outcome in 215 tumours of the extremity and trunk wall. Journal of Bone and Joint Surgery B. 2008;90(7):929–933. doi: 10.1302/0301-620X.90B7.20348. [DOI] [PubMed] [Google Scholar]
- 5.Evans HL. Atypical lipomatous tumor, its variants, and its combined forms: a study of 61 cases, with a minimum follow-up of 10 years. The American Journal of Surgical Pathology. 2007;31(1):1–14. doi: 10.1097/01.pas.0000213406.95440.7a. [DOI] [PubMed] [Google Scholar]
- 6.Mavrogenis AF, Lesensky J, Romagnoli C, et al. Atypical lipomatous tumors/well-differentiated liposarcomas: clinical outcome of 67 patients. Orthopedics. 2011;34:e893–e898. doi: 10.3928/01477447-20111021-11. [DOI] [PubMed] [Google Scholar]
- 7.Azumi N, Curtis J, Kempson RL, Hendrickson MR. Atypical and malignant neoplasms showing lipomatous differentiation: a study of 111 cases. The American Journal of Surgical Pathology. 1987;11(3):161–183. doi: 10.1097/00000478-198703000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Weiss SW, Rao VK. Well-differentiated liposarcoma (atypical lipoma) of deep soft tissue of the extremities, retroperitoneum, and miscellaneous sites: a follow-up study of 92 cases with analysis of the incidence of ‘dedifferentiation’. The American Journal of Surgical Pathology. 1992;16(11):1051–1058. doi: 10.1097/00000478-199211000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Lucas DR, Nascimento AG, Sanjay BK, Rock MG. Well-differentiated liposarcoma: the mayo clinic experience with 58 cases. The American Journal of Clinical Pathology. 1994;102(5):677–683. doi: 10.1093/ajcp/102.5.677. [DOI] [PubMed] [Google Scholar]
- 10.Serpell JW, Chen RY. Review of large deep lipomatous tumours. ANZ Journal of Surgery. 2007;77(7):524–529. doi: 10.1111/j.1445-2197.2007.04042.x. [DOI] [PubMed] [Google Scholar]
- 11.Laurino L, Furlanetto A, Orvieto E, Dei Tos AP. Well-differentiated liposarcoma (atypical lipomatous tumors) Seminars in Diagnostic Pathology. 2001;18(4):258–262. [PubMed] [Google Scholar]
- 12.Evans HL, Soule EH, Winkelmann RK. Atypical lipoma, atypical intramuscular lipoma, and well differentiated retroperitoneal liposarcoma: a reappraisal of 30 cases formerly classified as well differentiated liposarcoma. Cancer. 1979;43(2):574–584. doi: 10.1002/1097-0142(197902)43:2<574::aid-cncr2820430226>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 13.Rozental TD, Khoury LD, Donthineni-Rao R, Lackman RD. Atypical lipomatous masses of the extremities: outcome of surgical treatment. Clinical Orthopaedics and Related Research. 2002;(398):203–211. doi: 10.1097/00003086-200205000-00029. [DOI] [PubMed] [Google Scholar]
- 14.Bassett MD, Schuetze SM, Disteche C, et al. Deep-seated, well differentiated lipomatous tumors of the chest wall and extremities: the role of cytogenetics in classification and prognostication. Cancer. 2005;103(2):409–416. doi: 10.1002/cncr.20779. [DOI] [PubMed] [Google Scholar]
- 15.Sommerville SM, Patton JT, Luscombe JC, Mangham DC, Grimer RJ. Clinical outcomes of deep atypical lipomas (well-differentiated lipoma-like liposarcomas) of the extremities. ANZ Journal of Surgery. 2005;75(9):803–806. doi: 10.1111/j.1445-2197.2005.03519.x. [DOI] [PubMed] [Google Scholar]
- 16.Davis AM, O’Sullivan B, Turcotte R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiotherapy and Oncology. 2005;75(1):48–53. doi: 10.1016/j.radonc.2004.12.020. [DOI] [PubMed] [Google Scholar]


