An analysis of fungal phenology in a recent issue of PNAS suggested that there has been an extension of the mushroom fruiting season across Europe (1). The extended season effect is particularly strong in the United Kingdom (1, 2) and was attributed to greater climatic changes in that country. Here, we contend that the extended season conclusion may be correct but that the suggested mechanisms that contribute to it (1) are not so.
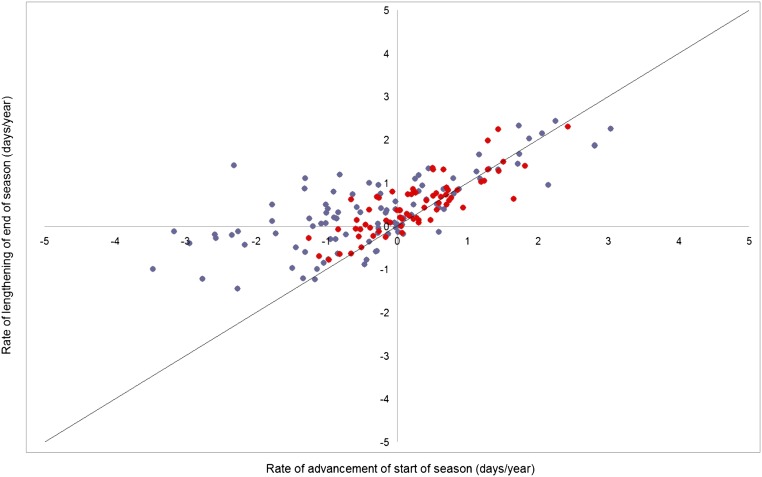
The recent analysis concentrated on saprotrophic and ectomycorrhizal fungi using a national database (1). An identical analysis of the same fungi species (1), and listed in an earlier, localized, and rigorous study in the United Kingdom (2), shows a very different pattern (Fig. 1). It should be noted that the local data were not included in the national set. In the national analysis, many species showed earlier first appearance and later last appearance (Fig. 1, upper left quadrant), whereas no mycorrhizal and only 6% of saprotrophs showed earlier first and last appearances (Fig. 1, lower left quadrant). In our local data, the latter figures are 21% and 33%, respectively. The critical difference lies in the end of the fruiting season, and we argue that the season extension in saprotrophs is mainly caused by first appearance advancing at a greater rate than last appearance. Furthermore, local data show that most mycorrhizal species show later fruiting seasons, in contrast to saprotrophs (Fig. 1).
Fig. 1.
Relationship between the start of fruiting season and end of season for saprotrophic (blue circles) and mycorrhizal (red circles) species in Gange et al. (2) and also listed in Kauserud et al. (1).
Kauserud et al. (1) contended that most fungi are continuing to fruit later in the year, which implies that higher temperatures allow continued fruiting, irrespective of resource availability. However, even in “warm” autumns, frosts still occur that cause fruiting to cease. We suggest that it is also resource availability that determines the disappearance of saprotrophic fruit bodies. As first appearance is advanced, so is the last fruiting date, because resources needed for basidiocarp production become depleted in the mycelium. Many mycorrhizas show later fruiting because resource availability from their tree hosts has become delayed.
Indeed, a “warming-induced” shift in fungal fruiting phenology was by no means justified by Kauserud et al. (1) but only implied: relationships with climatic factors were limited to a graphical presentation of air temperature variables, and no clear evidence of the influence (or not) of climate change was provided. Many other factors, such as habitat change, atmospheric deposition, and recorder behavior could easily account for the changes observed.
Analyses of national databases do not yield similar conclusions to local, more rigorous sampling procedures (3). Apart from the various sources of bias (4), a further problem is that the national analysis only covered the period of 1970–2007, whereas the local data cover 1950–2010. It is known that changes in the British flora and fungi mirror changes in climate that become apparent from the mid-1970s (2), so the shorter-term nature of the analysis (1) added a further source of bias, leading to potentially spurious conclusions (1, 5).
Footnotes
The authors declare no conflict of interest.
References
- 1.Kauserud H, et al. Warming-induced shift in European mushroom fruiting phenology. Proc Natl Acad Sci USA. 2012;109(36):14488–14493. doi: 10.1073/pnas.1200789109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gange AC, Gange EG, Sparks TH, Boddy L. Rapid and recent changes in fungal fruiting patterns. Science. 2007;316(5821):71. doi: 10.1126/science.1137489. [DOI] [PubMed] [Google Scholar]
- 3.Mattock G, Gange AC, Gange EG. Spring fungi are fruiting earlier. Brit Wildl. 2007;18(4):267–272. [Google Scholar]
- 4.Lobo JM. Database records as a surrogate for sampling effort provide higher species richness estimations. Biodivers Conserv. 2008;17(4):873–881. [Google Scholar]
- 5.Kauserud H, et al. Mushroom fruiting and climate change. Proc Natl Acad Sci USA. 2008;105(10):3811–3814. doi: 10.1073/pnas.0709037105. [DOI] [PMC free article] [PubMed] [Google Scholar]