Fig. P1.
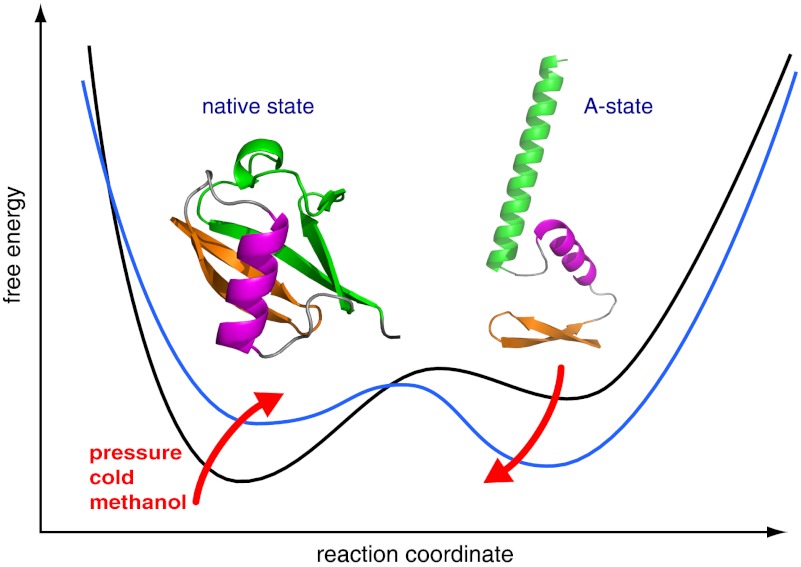
High-resolution NMR experiments show that the free energy landscape of ubiquitin changes under cold and pressure denaturation in a similar way as under alcohol denaturation. These conditions cause an opening of the structure and induce a structural ensemble where certain parts of native secondary structure are preserved whereas others change to nonnative forms. These individual secondary structure elements undergo independent segmental motions.