Abstract
Chronic kidney disease (CKD) is a major cause of death and morbidity in Australia and worldwide. DNA vaccination has been used for targeting foreign antigens to induce immune responses and prevent autoimmune disease, viral infection and cancer. However, the use of DNA vaccination has been restricted by a limited ability to induce strong immune responses, especially against self-antigens which are limited by mechanisms of self-tolerance. Furthermore, there have been few studies on the potential of DNA vaccination in chronic inflammatory diseases, including CKD. We have established strategies of DNA vaccination targeting specific self-antigens in the immune system including co-stimulatory pathways, T cell receptors and chemokine molecules, which have been effective in protecting against the development of CKD in a variety of animal models. In particular, we find that the efficacy of DNA vaccination is improved by dendritic cell (DC) targeting and can protect against animal models of autoimmune nephritis mimicking human membranous nephropathy. In this review, we summarize several approaches that have been tested to improve the efficacy of DNA vaccination in CKD models, including enhanced DNA vaccine delivery methods, DNA vaccine modifications and new molecular targets for DNA vaccination. Finally, we discuss the specific application of DNA vaccination for preventing and treating CKD.
Keywords: DNA vaccination, dendritic cell, DEC205, CD40, cytokine, costimulatory molecular, active Heymann nephritis (HN), adriamycin nephropathy (AN)
Introduction
Chronic kidney disease (CKD) is characterized by the progressive loss of renal function and structural injury leading eventually to end stage kidney disease (ESKD) [1,2]. Currently, treatment strategies that successfully delay progression from CKD to ESKD are limited and patients with ESKD require costly dialysis or renal transplantation. Therefore, new strategies to manage CKD are important from both a clinical and public health perspective. DNA vaccination delivers plasmid DNA encoding the target gene to induce both humoral and cellular immune responses. This strategy has been used for more than two decades to treat autoimmune disease, viral infection and cancer [3-7]. While DNA vaccines have reached clinical use, in general they have been limited by their restricted ability to induce strong immune responses [8] and this is a particular problem in generating responses to self-antigens where there is intrinsic self-tolerance [3,9]. In addition, the potential of DNA vaccination as therapeutic approach for CKD has not been assessed fully. Our previous studies have shown that DNA vaccination targeting T cell receptor (TCR) subsets in Heymann nephritis (HN) [10], or targeting the chemokine CCL2 (monocyte chemoattractant protein 1) in Adriamycin nephropathy (AN) are protective and induce specific cellular and antibody responses against the target antigen [11,12]. A number of strategies have been utilized to enhance efficacy. We have recently tested a plasmid containing the gene encoding a single-chain Fv antibody specific for the dendritic cell-restricted antigen-uptake receptor DEC205 developed by the Steinman laboratory. By cloning a gene of interest, co-stimulatory molecule CD40, into this plasmid, we have demonstrated that this particular vaccine (DEC205-CD40) can prevent the development of HN, a rat model of human membranous nephropathy (KI 2012). More broadly DC targeted vaccines against other chemokine targets such as CX3CR1 induce functional antibody responses against self-antigens [13].
We have called this induction of immune responses against targeted self antigens “daedalic in reference to the Greek myth of Daedalus who induced self-injury while flying to close to the sun.
Similar to standard vaccines, DNA vaccines are believed to confer protection through neutralizing antibodies. Our previous studies suggest that DNA vaccination results in antigen-specific antibody responses that can be measured by enzyme-linked immunosorbent assays, as well as assays that measure functional antibody activity. We have found that enhanced antigen specific T cell responses using the the tetanus toxoid element p30 in our plasmid enhances antigen specific T cell responses as measured by ELISPOT and provide additional T cell help for antibody production [12].
Improvement of efficacy of DNA vaccine by modification of vaccine and delivery methods
There are several ways of delivering a plasmid DNA into target cells. One of the most commonly used delivery methods is intramuscular injection. However, intramuscular injection is often not enough to elicit a strong immune response in rodents. Various approaches have therefore been adopted to improve the immunogenicity of DNA vaccines such as the prime-boost immunization of protein antigen in adjuvant and the use of modified DNA delivery systems including electroporation [14-16]. Our previous studies have shown that the administration of antigens (recombinant proteins or peptides) in Complete Freund’s Adjuvant (CFA) after injection of plasmid DNA encoding the target antigen enhance the immunogenicity of the vaccine in mice. CFA is composed of dried or inactivated mycobacterial components and itself is an immunopotentiator of cell-mediated immunity and production of antibodies by stimulating TNF-α production by APCs [17]. We have also demonstrated that fusion of the gene encoding the tetanus toxoid T helper epitope P30 with target genes is able to increase the immunogenicity of DNA vaccines by engaging T cell help [12].
Electroporation is another useful delivery approach which enhances the efficacy of DNA vaccines by facilitating plasmid entry into target cells [18]. Delivery of short pulses to the injection site causes temporary permeabilization of the cell membrane, thereby facilitating DNA uptake. An increased antigen expression in mice, guinea pigs and rabbits has been observed previously with the use of electroporation [18,19]. Studies have suggested that applying an electric field to tissues in vivo significantly increase DNA uptake and gene expression [7,18]. We and others have also found that electroporation substantially increases DNA delivery and DNA vaccine potency in animal models of CKD.
Enhancement of DNA vaccination by targeting the encoded protein to dendritic cells (DCs)
One of the most promising methods for enhancing the efficacy of DNA vaccination is the selective targeting of DNA vaccine encoded antigens to the immune cells, especially antigen presenting cells (APCs). This is due to the requirement for MHC Class I and II expression [20] and the expression of co-stimulatory molecules, particularly CD80 and CD86 which are important for the efficient antigen processing, presentation and induction of T cell immune responses. DCs have been identified as the most potent APCs that can prime T cells in vivo [21]. DC targeting can be achieved by the use of DEC205 ScFv antibody constructs in the plasmid. These encode a fusion protein comprised of the vaccine antigen and a single-chain Fv antibody (scFv) specific for the DC-restricted antigen-uptake receptor DEC205. DEC205 targeted DNA vaccines substantially increase antibody production and cellular responses [20,22]. As showed in Figure 1, antigen encoding sequence is incorporated into a DC-targeted DNA plasmid which contains scFv sequence encoding for an antibody directed at DEC205, then the plasmid DNA is delivered intramuscularly with electroporation and taken up by DCs. DCs migrate into lymph nodes where they differentiate and induce T cell and B cell responses. Finally, antibody production and cellular response is induced by DC-targeted DNA vaccination.
Figure 1.
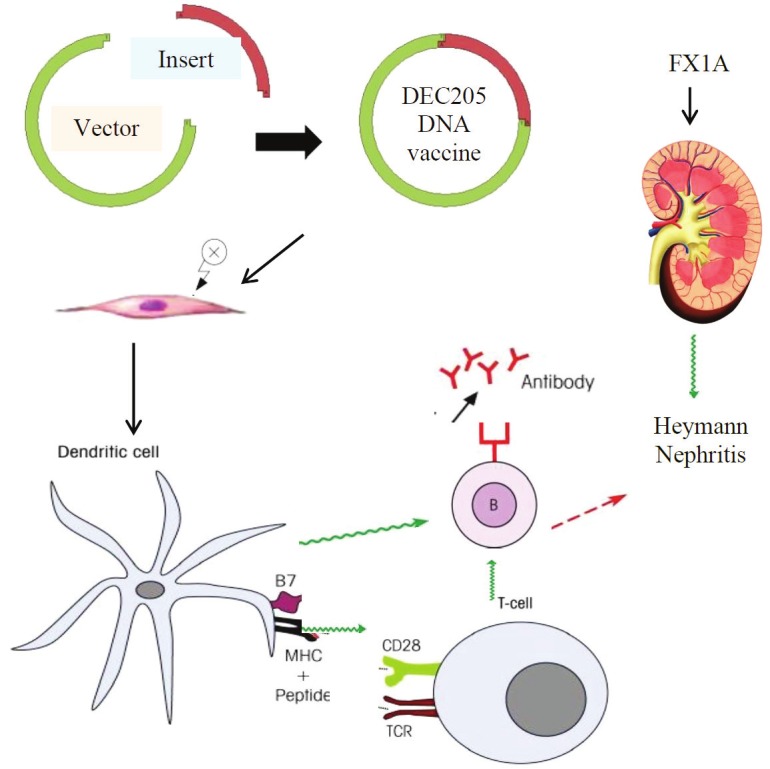
Immunological mechanisms of DNA vaccination in preventing Heymann Nephritis. Antigen encoding sequence is incorporated into a DC-targeted DNA plasmid which contains a scFv sequence encoding for antibodies directed against DEC205. Plasmid DNAs are delivered intramuscularly with electroporation and taken up by DCs. DCs migrate into lymph nodes where they differentiate and induce T cell and B cell responses. B cells differentiate into plasma cells and produce antigen-specific neutralizing antibodies limiting immune activation in chronic kidney disease.
DCs generate specific adaptive immunity against the vaccinated antigens or antigenic epitopes by augmenting T-cell mediated responses. After uptake of antigens, DCs migrate to secondary lymphoid organs where they process and present the antigens to naive T cells via MHC-I or II molecules inducing activation of antigen-specific T cells [23]. Antigen-specific T cells include CD4+ T cells and CD8+ T cells. of CD4+ T cell subsets including the newly described T follicular helper cells secrete cytokines including IL-4 and IL-21 that induces B cell differentiation and lead to the production of protective neutralizing antibodies and formation of plasma and memory B cells [24]. Both CD4+ and CD8+ T cells can undergo further differentiation and become memory cells [25].
There has been a relatively simplistic model of Th1 and Th2 differentiation driving different immune responses generated by DNA vaccines [26]. It has been postulated that, IL-12 or IL-4 can drive Th1 or Th2 cell development respectively [27,28]. Th2 responses further activate B cells to become antibody-secreting plasma cells leading to humoral responses [29] and that the type of T helper response induced is related to the method of DNA vaccine delivery, target sites and nature of immunogens. For example, needle injection (IM or ID) can induce a Th1 response while the gene gun method induces Th2 responses [30,31]. Our results are different from this and we have found increased immunogenicity and antibody levels associated with IFN-γ secretion and Th1 formation with the addition of adjuvants such as P30. This may reflect the need for some form of inflammation to break self-tolerance [11-13].
In standard DNA vaccination against pathogens an important advantage of DNA vaccination is its capability of raising CTL (CD8+ effector cytotoxic T-lymphocyte) responses. CTL responses are activated by differentiated DCs through the MHC-I priming pathway and further augmented by CD4+ T helper cells [29]. DNA vaccines that target the MHC-I restricted pathway via direct or cross priming of antigens greatly enhanced CTL responses in animal models [32,33]. This was further emphasized recently with the development of a DNA vaccine encoding antigen peptide combined with an retention signal which targeted intercellular trafficking of MHC-I molecule presentation and produced significant CTL responses in mice with marked production of IFN-γ [34]. Furthermore, several studies had revealed that DNA vaccines against infectious agents such as HBV, influenza, and HIV strongly promote the CD8+ responses [23,35,36]. This differs from our studies against self antigens where the major aim is to induce blocking antibody responses.
Augmentation of immunity by DC-targeting DNA vaccination strategy has been tested in a number of disease models. It was previously shown that both a protective CD4+ T helper response and a CD8+ functional response were generated after vaccination of a recombinant gag virus vaccine against HIV in a mouse model with DC-targeting [37,38]. More recent studies focused on the utilization of specific viral vectors to suppress cancers and preventing the development of HIV in mouse models. Animals that received DC-targeted vaccine composed of modified adenoviral vectors generated significantly more CD8+ cells (including CD62L-/CD127+ effector memory cells), produced higher levels of IL-2 and were protected from melanoma tumor growth [39]. In another study, DNA vaccination using DC-targeted recombinant Newcastle disease virus (rNDV) vectors induced significant interferon production and generated HIV-gag antigen specific humoral and CD4+/CD8+ T cell responses in mice [40]. Finally primate studies have demonstrated a 10-fold increase of immunogenicity in rhesus macaques after immunization with DNA vaccines encoding simian immunodeficiency virus antigen targeted to dendritic cells [41].
Autoimmune kidney disease targeted by DNA vaccination
Chronic kidney disease in humans and in mouse models involves both the cognate and innate immune systems and can be targeted at the levels of activation, differentiation and trafficking. We have shown that blockade of CD40L improves renal outcomes in models of progressive kidney disease [10,54]. Strategies targeting either specific effector cells or the mode by which they are recruited to sites of inflammation may allow highly specific interventions that are long lasting, robust and do not have significant side effects.
Because of the clinical problems with CD40L as a clinical target due to thrombosis induced through CD40L on platelets we have focused on its ligand CD40. Many of the co-stimulatory pathways contain key molecules for therapeutic intervention including some such as belatacept which blocks CD28 activation, which have reached clinical use. CD40 is expressed by B cells as well as other APCs and its ligand CD154 which is expressed widely on T cells is a critical co-stimulatory pathway for T cell activation and the differentiation as well as class switching of B cells [42]. Blockade of CD40-CD154 is protective in a number of renal disease models such as rodent membranous glomerulonephritis, chronic proteinuric renal disease and Adriamycin nephropathy (AN) [43-45]. Recent studies have demonstrated that CD40 and CD154 neutralizing antibodies are highly effective in blocking this pathway by generating antigen specific Tregs and limiting antigen specific CD8 expansion [46], and their use has reached the stage of preclinical testing. Recent work by Steinman and others has shown the benefits of targeting DNA encoded immunogens to DCs in situ using scFv antibodies directed at DEC205 on the DC surface [22]. We have utilized this approach in targeting CD40 to DCs. The incorporation of a DC targeting element into a DNA vaccine allows the selective expression/uptake of vaccine encoded antigen by DC, which is critical for increased efficacy of MHC class II antigen presentation inducing an immune response to CD40 generating blocking antibodies that protect against membranous glomerulonephritis.
Chemokine and chemokine receptors: CCL2, CX3CR1
Recently there has been an increased interest and progress in research on DNA vaccination targeting small immune biomolecules including T cell receptors CRs, cytokines and chemokines in chronic inflammatory diseases. Chemokines (chemotactic cytokine) are a family of small molecules that play an important role in inducing chemotaxis and coordinating leukocyte trafficking during an inflammatory response [47]. Fractalkine (CX3CL1), CCL2 (Monocyte chemoattractant protein 1, MCP-1) and their receptors CX3CR1 and CCR2 are two important chemokine/receptor pairs which have been identified as contributing significantly to monocyte recruitment. Their involvement in clinical diseases such as atherosclerosis, nephropathy, rheumatoid arthritis, allograft rejection and various types of cancer is widely evident [48-53]. Previous studies from our laboratories and collaborators have demonstrated that DNA vaccination against TCR subsets in Heymann nephritis, and against the chemokine CCL2 in Adriamycin nephropathy is protective and induce specific cellular and antibody responses against the target antigen [10-12]. We have recently demonstrated that a DC-targeted DNA vaccine against CX3CR1 and CCL2 successfully induces humoral and cellular responses in mice. In this model, the generated autoantibodies restrict the motility of macrophages towards activated endothelial cells shown by in vitro functional analysis [13]. These findings suggest a potential therapeutic role of chemokine/receptor DNA vaccination in preventing inflammatory diseases. Studies of DNA vaccination for CKD models are summarized in Table 1.
Table 1.
DNA vaccination studies in kidney disease
| Vaccine Target | Vaccine delivery method | Modification | Disease Model | Immunogenicity | Prevention of Disease |
|---|---|---|---|---|---|
| TCRs | bupivacaine pretreatment | Heymann nephritis (HN) in rats | production of autoantibody | reduced proteinuria | |
| intramuscular injection | reduced macrophage, T cells infiltration | ||||
| challenged with Fx1A | reduced IFN- γ production | ||||
| CCL2 | bupivacaine pretreatment | P30 tetanus toxoid helper epitope sequence | Adriamycin Nephropathy (AN) in rats | production of Anti-CCL2 Ab | reduced glomerular and tubular damage |
| intramuscular injection | increased IFN-γ producing T cells | protected renal function | |||
| reduced glomerular and interstitial macrophage Infiltration | |||||
| CD40 | bupivacaine pretreatment | P30 tetanus toxoid helper epitope sequence | Adriamycin Nephropathy (AN) in rats | production of anti-CD40 | protected renal functions |
| intramuscular injection | DC-targeting | reduced renal structural injury | |||
| electroporation | reduced macrophage, T cells infiltration and IgG deposition | ||||
| challenged with Fx1A |
Therapeutic applications for human renal diseases
Clinical application of DNA vaccines has occurred in viral infection, cancer and autoimmune diseases in recent years. The success of DNA vaccines against multiple strains of influenza, human papillomavirus and HIV-1 in both preclinical models and clinical trials is promising [8]. However, potency and safety of DNA vaccines remain the major challenges to their application and had the major limitations in clinical applications. The ability to deliver vaccines better though gene guns and direct them to DCs allows more specific therapy without the need to increase adjuvants are major advances. While potency is a requirement for human studies, there are also concerns regarding the use of adjuvants in chronic inflammatory conditions and of inducing potentially lifelong blockade of specific pathways in the immune system. However the results in animal studies are encouraging that specifically targeting self antigens through DNA vaccination (Daedelic vaccination) can be made potent enough to deliver clinical benefits. What remains to be tested is whether these beneifts can be extended to human.
Conclusions
The new approaches described to improve DNA vaccination induce more potent cellular and humoral response and present a potential preventative and therapeutic strategy for a variety of autoimmune diseases. Co-stimulatory molecules and chemokines are important therapeutic targets for DNA vaccination to treat CKD. Modulation of DNA vaccination with adjuvant and DC targeting improves efficacy without increasing toxicity during the treatment for CKD. Further studies are rquired to explore combining DNA vaccination approaches with enhanced methods for delivery, modified adjuvants and new molecular gene targets. The induction of self-immune responses to block key pathways reflects many natural mechanisms of self-regulation and may offer potent therapeutic strategies for the treatment of CKD.
References
- 1.Lee G. End-stage renal disease in the Asian-Pacific region. Semin Nephrol. 2003;23:107–114. doi: 10.1053/snep.2003.50009. [DOI] [PubMed] [Google Scholar]
- 2.Fogo AB. Mechanisms of progression of chronic kidney disease. Pediatr Nephrol. 2007;22:2011–2022. doi: 10.1007/s00467-007-0524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ada G, Ramshaw I. DNA vaccination. Expert Opin Emerg Drugs. 2003;8:27–35. doi: 10.1517/14728214.8.1.27. [DOI] [PubMed] [Google Scholar]
- 4.Silva CL, Bonato VL, dos Santos-Junior RR, Zarate-Blades CR, Sartori A. Recent advances in DNA vaccines for autoimmune diseases. Expert Rev Vaccines. 2009;8:239–252. doi: 10.1586/14760584.8.2.239. [DOI] [PubMed] [Google Scholar]
- 5.Kim CY, Kang ES, Kim SB, Kim HE, Choi JH, Lee DS, Im SJ, Yang SH, Sung YC, Kim BM, Kim BG. Increased in vivo immunological potency of HB-110, a novel therapeutic HBV DNA vaccine, by electroporation. Exp Mol Med. 2008;40:669–676. doi: 10.3858/emm.2008.40.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houot R, Kohrt HE, Marabelle A, Levy R. Targeting immune effector cells to promote antibody-induced cytotoxicity in cancer immunotherapy. Trends Immunol. 2011 Nov;32:510–6. doi: 10.1016/j.it.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Iezzi M, Quaglino E, Amici A, Lollini PL, Forni G, Cavallo F. DNA vaccination against onco-antigens: A promise. Oncoimmunology. 2012;1:316–325. doi: 10.4161/onci.19127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferraro B, Morrow MP, Hutnick NA, Shin TH, Lucke CE, Weiner DB. Clinical Applications of DNA Vaccines: Current Progress. Clin Infect Dis. 2011;53:296–302. doi: 10.1093/cid/cir334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ada G. Vaccines and vaccination. N Engl J Med. 2001;345:1042–1053. doi: 10.1056/NEJMra011223. [DOI] [PubMed] [Google Scholar]
- 10.Wu H, Walters G, Knight JF, Alexander SI. DNA vaccination against specific pathogenic TCRs reduces proteinuria in active Heymann nephritis by inducing specific autoantibodies. J Immunol. 2003;171:4824–4829. doi: 10.4049/jimmunol.171.9.4824. [DOI] [PubMed] [Google Scholar]
- 11.Wu H, Wang Y, Tay YC, Zheng G, Zhang C, Alexander SI, Harris DC. DNA vaccination with naked DNA encoding MCP-1 and RANTES protects against renal injury in adriamycin nephropathy. Kidney Int. 2005;67:2178–2186. doi: 10.1111/j.1523-1755.2005.00323.x. [DOI] [PubMed] [Google Scholar]
- 12.Zheng G, Wang Y, Xiang SH, Tay YC, Wu H, Watson D, Coombes J, Rangan GK, Alexander SI, Harris DC. DNA vaccination with CCL2 DNA modified by the addition of an adjuvant epitope protects against “nonimmune” toxic renal injury. J Am Soc Nephrol. 2006;17:465–474. doi: 10.1681/ASN.2005020164. [DOI] [PubMed] [Google Scholar]
- 13.Zhou JJ, Wang YM, Lee VW, Phoon RK, Zhang GY, Wang Y, Tan TK, Hu M, Wang LD, Saito M, Sawyer A, Harris DCH, Alexander SI, Durkan AM. DEC205-DC targeted DNA vaccines to CX3CR1 and CCL2 are potent and limit macrophage migration. Int J Clin Exp Med. 2012;5:24–33. [PMC free article] [PubMed] [Google Scholar]
- 14.Coban C, Kobiyama K, Aoshi T, Takeshita F, Horii T, Akira S, Ishii KJ. Novel strategies to improve DNA vaccine immunogenicity. Curr Gene Ther. 2011;11:479–484. doi: 10.2174/156652311798192815. [DOI] [PubMed] [Google Scholar]
- 15.Moore AC, Hill AV. Progress in DNA-based heterologous prime-boost immunization strategies for malaria. Immunol Rev. 2004;199:126–143. doi: 10.1111/j.0105-2896.2004.00138.x. [DOI] [PubMed] [Google Scholar]
- 16.Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol. 2011;23:421–429. doi: 10.1016/j.coi.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oikawa Y, Shimada A, Yamada Y, Okubo Y, Katsuki T, Shigihara T, Miyazaki J, Narumi S, Itoh H. CXC chemokine ligand 10 DNA vaccination plus Complete Freund’s Adjuvant reverses hyperglycemia in non-obese diabetic mice. Rev Diabet Stud. 2010;7:209–224. doi: 10.1900/RDS.2010.7.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Widera G, Austin M, Rabussay D, Goldbeck C, Barnett SW, Chen M, Leung L, Otten GR, Thudium K, Selby MJ, Ulmer JB. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J Immunol. 2000;164:4635–4640. doi: 10.4049/jimmunol.164.9.4635. [DOI] [PubMed] [Google Scholar]
- 19.Mathiesen I. Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Ther. 1999;6:508–514. doi: 10.1038/sj.gt.3300847. [DOI] [PubMed] [Google Scholar]
- 20.Wang G, Pan L, Zhang Y. Approaches to improved targeting of DNA vaccines. Hum Vaccin. 2011;7:1271–1281. doi: 10.4161/hv.7.12.17983. [DOI] [PubMed] [Google Scholar]
- 21.Su JH, Wu A, Scotney E, Ma B, Monie A, Hung CF, Wu TC. Immunotherapy for cervical cancer: Research status and clinical potential. BioDrugs. 2010;24:109–129. doi: 10.2165/11532810-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nchinda G, Kuroiwa J, Oks M, Trumpfheller C, Park CG, Huang Y, Hannaman D, Schlesinger SJ, Mizenina O, Nussenzweig MC, Uberla K, Steinman RM. The efficacy of DNA vaccination is enhanced in mice by targeting the encoded protein to dendritic cells. J Clin Invest. 2008;118:1427–1436. doi: 10.1172/JCI34224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12:509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 25.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson HL, Boyle CA, Feltquate DM, Morin MJ, Santoro JC, Webster RG. DNA immunization for influenza virus: studies using hemagglutinin- and nucleoprotein-expressing DNAs. J Infect Dis. 1997;176(Suppl 1):S50–55. doi: 10.1086/514176. [DOI] [PubMed] [Google Scholar]
- 27.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 28.Jakob T, Walker PS, Krieg AM, Udey MC, Vogel JC. Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides: a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J Immunol. 1998;161:3042–3049. [PubMed] [Google Scholar]
- 29.Lin K, Roosinovich E, Ma B, Hung CF, Wu TC. Therapeutic HPV DNA vaccines. Immunol Res. 2010;47:86–112. doi: 10.1007/s12026-009-8141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alarcon JB, Waine GW, McManus DP. DNA vaccines: technology and application as anti-parasite and anti-microbial agents. Adv Parasitol. 1999;42:343–410. doi: 10.1016/s0065-308x(08)60152-9. [DOI] [PubMed] [Google Scholar]
- 31.Feltquate DM, Heaney S, Webster RG, Robinson HL. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J Immunol. 1997;158:2278–2284. [PubMed] [Google Scholar]
- 32.Cho JH, Youn JW, Sung YC. Cross-priming as a predominant mechanism for inducing CD8(+) T cell responses in gene gun DNA immunization. J Immunol. 2001;167:5549–5557. doi: 10.4049/jimmunol.167.10.5549. [DOI] [PubMed] [Google Scholar]
- 33.Kim TW, Hung CF, Boyd D, Juang J, He L, Kim JW, Hardwick JM, Wu TC. Enhancing DNA vaccine potency by combining a strategy to prolong dendritic cell life with intracellular targeting strategies. J Immunol. 2003;171:2970–2976. doi: 10.4049/jimmunol.171.6.2970. [DOI] [PubMed] [Google Scholar]
- 34.Isaji K, Kawase A, Matono M, Guan X, Nishikawa M, Takakura Y. Enhanced CTL response by controlled intracellular trafficking of antigen in dendritic cells following DNA vaccination. J Control Release. 2009;135:227–233. doi: 10.1016/j.jconrel.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 35.Du X, Zhao B, Li J, Cao X, Diao M, Feng H, Chen X, Chen Z, Zeng X. Astragalus polysaccharides enhance immune responses of HBV DNA vaccination via promoting the dendritic cell maturation and suppressing Treg frequency in mice. Int Immunopharmacol. 2012;14:463–470. doi: 10.1016/j.intimp.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Shen X, Soderholm J, Lin F, Kobinger G, Bello A, Gregg DA, Broderick KE, Sardesai NY. Influenza A vaccines using linear expression cassettes delivered via electroporation afford full protection against challenge in a mouse model. Vaccine. 2012 Nov 6;30:6946–54. doi: 10.1016/j.vaccine.2012.02.071. [DOI] [PubMed] [Google Scholar]
- 37.Trumpfheller C, Caskey M, Nchinda G, Longhi MP, Mizenina O, Huang Y, Schlesinger SJ, Colonna M, Steinman RM. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc Natl Acad Sci U S A. 2008;105:2574–2579. doi: 10.1073/pnas.0711976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nchinda G, Amadu D, Trumpfheller C, Mizenina O, Uberla K, Steinman RM. Dendritic cell targeted HIV gag protein vaccine provides help to a DNA vaccine including mobilization of protective CD8+ T cells. Proc Natl Acad Sci U S A. 2010;107:4281–4286. doi: 10.1073/pnas.1000621107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tenbusch M, Nchinda G, Storcksdieck Genannt Bonsmann M, Temchura V, Uberla K. Targeting the antigen encoded by adenoviral vectors to the DEC205 receptor modulates the cellular and humoral immune response. Int Immunol. 2012 Nov 26; doi: 10.1093/intimm/dxs112. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Maamary J, Array F, Gao Q, Garcia-Sastre A, Steinman RM, Palese P, Nchinda G. Newcastle disease virus expressing a dendritic cell-targeted HIV gag protein induces a potent gagspecific immune response in mice. J Virol. 2011;85:2235–2246. doi: 10.1128/JVI.02036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tenbusch M, Ignatius R, Nchinda G, Trumpfheller C, Salazar AM, Topfer K, Sauermann U, Wagner R, Hannaman D, Tenner-Racz K, Racz P, Stahl-Hennig C, Uberla K. Immunogenicity of DNA vaccines encoding simian immunodeficiency virus antigen targeted to dendritic cells in rhesus macaques. PLoS One. 2012;7:e39038. doi: 10.1371/journal.pone.0039038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diehl L, Den Boer AT, van der Voort EI, Melief CJ, Offringa R, Toes RE. The role of CD40 in peripheral T cell tolerance and immunity. J Mol Med (Berl) 2000;78:363–371. doi: 10.1007/s001090000126. [DOI] [PubMed] [Google Scholar]
- 43.Biancone L, Andres G, Ahn H, DeMartino C, Stamenkovic I. Inhibition of the CD40-CD40 ligand pathway prevents murine membranous glomerulonephritis. Kidney Int. 1995;48:458–468. doi: 10.1038/ki.1995.314. [DOI] [PubMed] [Google Scholar]
- 44.Kairaitis L, Wang Y, Zheng L, Tay YC, Harris DC. Blockade of CD40-CD40 ligand protects against renal injury in chronic proteinuric renal disease. Kidney Int. 2003;64:1265–1272. doi: 10.1046/j.1523-1755.2003.00223.x. [DOI] [PubMed] [Google Scholar]
- 45.Reynolds J, Khan SB, Allen AR, Benjamin CD, Pusey CD. Blockade of the CD154-CD40 costimulatory pathway prevents the development of experimental autoimmune glomerulonephritis. Kidney Int. 2004;66:1444–1452. doi: 10.1111/j.1523-1755.2004.00907.x. [DOI] [PubMed] [Google Scholar]
- 46.Ferrer IR, Wagener ME, Song M, Kirk AD, Larsen CP, Ford ML. Antigen-specific induced Foxp3+ regulatory T cells are generated following CD40/CD154 blockade. Proc Natl Acad Sci U S A. 2011;108:20701–20706. doi: 10.1073/pnas.1105500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D’Haese JG, Demir IE, Friess H, Ceyhan GO. Fractalkine/CX3CR1: why a single chemokine-receptor duo bears a major and unique therapeutic potential. Expert Opin Ther Targets. 2010;14:207–219. doi: 10.1517/14728220903540265. [DOI] [PubMed] [Google Scholar]
- 48.Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 49.Hara A, Wada T. [Role of chemokines/chemokine receptors in pathogenesis of diabetic nephropathy] . Nihon Jinzo Gakkai Shi. 2011;53:1027–1033. [PubMed] [Google Scholar]
- 50.Pavkova Goldbergova M, Lipkova J, Pavek N, Gatterova J, Vasku A, Soucek M, Nemec P. RANTES, MCP-1 chemokines and factors describing rheumatoid arthritis. Mol Immunol. 2012;52:273–278. doi: 10.1016/j.molimm.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Haskell CA, Hancock WW, Salant DJ, Gao W, Csizmadia V, Peters W, Faia K, Fituri O, Rottman JB, Charo IF. Targeted deletion of CX(3)CR1 reveals a role for fractalkine in cardiac allograft rejection. J Clin Invest. 2001;108:679–688. doi: 10.1172/JCI12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pienta KJ, Machiels JP, Schrijvers D, Alekseev B, Shkolnik M, Crabb SJ, Li S, Seetharam S, Puchalski TA, Takimoto C, Elsayed Y, Dawkins F, de Bono JS. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Invest New Drugs. 2012 Aug 21; doi: 10.1007/s10637-012-9869-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 53.Vitale S, Cambien B, Karimdjee BF, Barthel R, Staccini P, Luci C, Breittmayer V, Anjuere F, Schmid-Alliana A, Schmid-Antomarchi H. Tissue-specific differential antitumour effect of molecular forms of fractalkine in a mouse model of metastatic colon cancer. Gut. 2007;56:365–372. doi: 10.1136/gut.2005.088989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Wang YM, Zheng G, Zhang GY, Zhou JJ, Tan TK, Cao Q, Hu M, Watson D, Wu H, Zheng D, Wang C, Lahoud MH, Caminschi I, Harris DC, Alexander SI. DNA vaccine encoding CD40 targeted to dendritic cells in situ prevents the development of Heymann nephritis in rats. Kidney Int. 2012 doi: 10.1038/ki.2012.374. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


