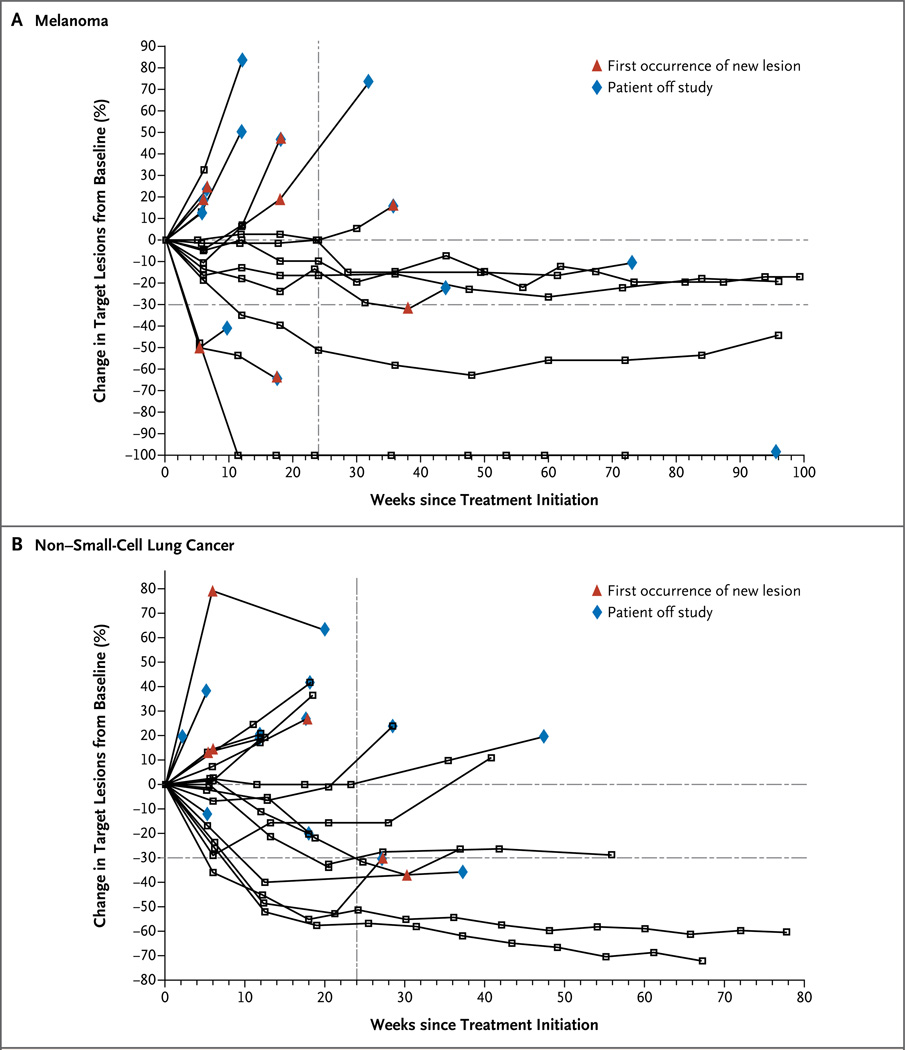
Figure 1. Activity of Anti–PD-L1 Antibody in Patients with Advanced Melanoma and Non–Small-Cell Lung Cancer.
Shown is the tumor burden (assessed as the longest linear dimension) over time in patients with melanoma (Panel A) and non–small-cell lung cancer (Panel B) who received 10 mg of anti–PD-L1 antibody per kilogram of body weight. In most patients who had an objective response, responses were durable and were evident by the end of cycle 2 (12 weeks) of treatment, regardless of the drug dose or tumor type. The vertical dashed line marks the 24-week time point at which the rate of progression-free survival was calculated. Tumor regression followed both conventional and immune-related patterns of response, such as a prolonged reduction in the tumor burden in the presence of new lesions.