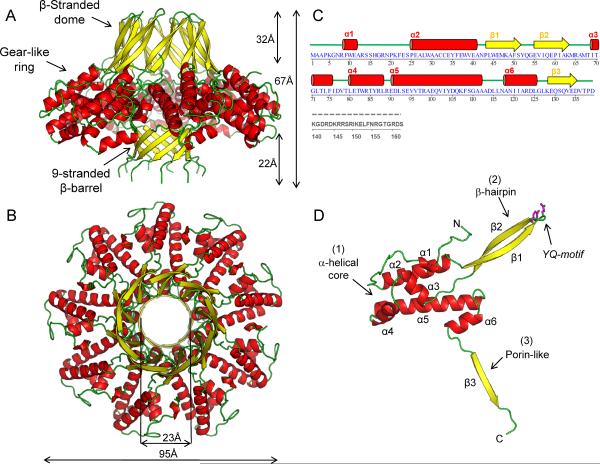
Figure 1. Quaternary structure of the nonameric S-terminase subunit of bacteriophage P22.
Ribbon diagram of S-terminase in side (A) and top (B) views. The oligomer is colored by secondary structure elements with α-helices, β-strands and loops in red, yellow and green, respectively. The overall diameter of S-terminase is ~95 Å with an internal hollow channel ~23 Å. (C) Secondary structure and amino acid sequence of bacteriophage P22 S-terminase subunit. Dashed in gray is the DNA-binding domain spanning residues 140–162, which is proteolytically cleaved in the 1.75 Å structure used for high resolution refinement and is disordered in the 3.35 Å structure of fl-S-terminase (Figure S1B). The illustration was generated using STRIDE (Heinig and Frishman, 2004). (D) Ribbon diagram of S-terminase protomer colored as in (A). The side chains for the YQ-motif on the tip of S-terminase are shown as sticks.