Figure 1.
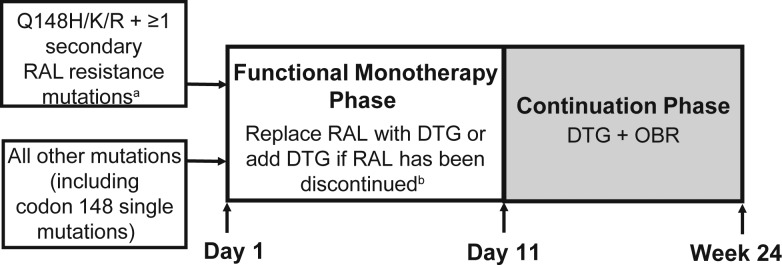
Study design. Subjects received dolutegravir (DTG) 50 mg once daily (cohort I) or DTG 50 mg twice daily (cohort II). In both cohorts, subjects were allocated to 1 of 2 groups on the basis of integrase genotype at screening, to ensure a broad representation of genotypes and a range of phenotypic susceptibility. Cohort II subjects were required to have ≥1 fully active antiretroviral therapy in an optimized background regimen (OBR). Abbreviation: RAL, raltegravir. aQ148H/K/R plus changes in L74, E138, or G140. bSubjects remained on failing background regimen from day 1 to day 10.
