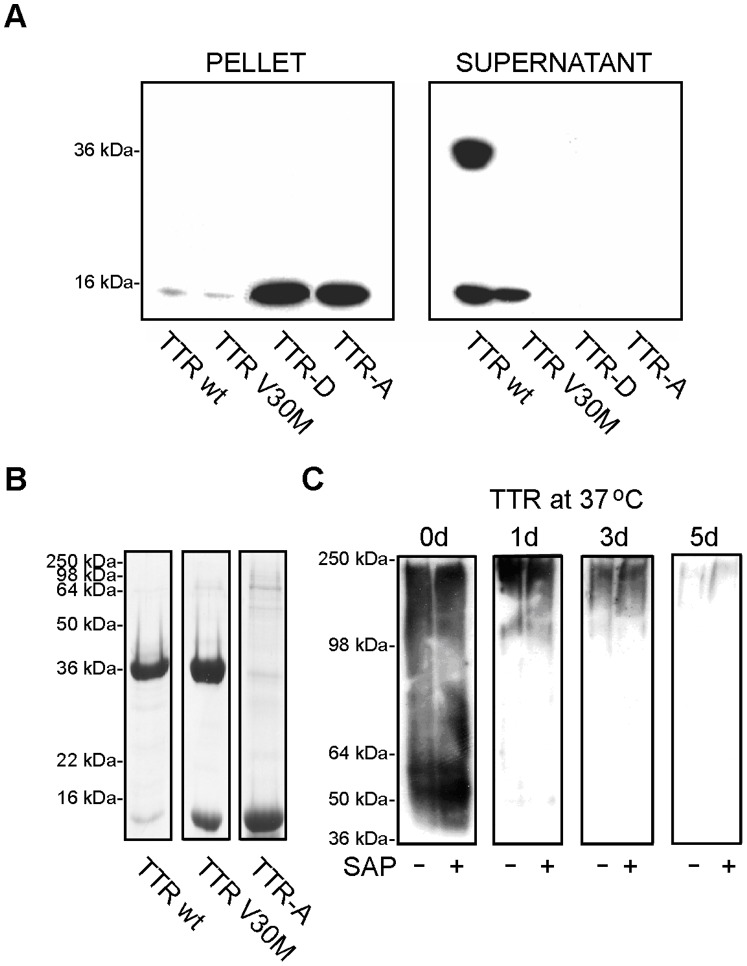
Figure 1. SAP binds to pre-fibrillar aggregates of TTR in vitro.
(A) SAP was co-incubated with pre-aggregated TTR under physiological conditions. The complexes were immunoprecipitated with a SAP-specific antibody (DAKO) and the presence of TTR was detected on immunoblots using a polyclonal anti-TTR antibody (DAKO). SAP bound to pre-fibrillar aggregates of TTR-D and TTR-A, and the precipitates were found in the pellet fraction (left panel), whereas TTR wt and TTR V30M were found unbound in the supernatants (right panel). Bands: 16 kDa–monomer; 36 kDa–dimer. (B) SDS-PAGE analysis of TTR variants. Immunoblot shows that the TTR-A mutant is sensitive to SDS and easily dissociates into monomers in contrast to TTRwt or TTRV30M that keep the dimers intact. (C) Effect of SAP on aggregation of TTR. The TTR-A mutant was aggregated at 37°C for 0–5 days in the presence (+) or absence (−) of 3 µM SAP and subjected to immunoblotting under native conditions. TTR was detected with a TTR-specific antibody. SAP did not affect the aggregation kinetics of the TTR-A mutant, since the migration pattern of TTR-A in the gel decreased with time as the protein formed higher-molecular-weight aggregates–and was identical irrespective of whether or not SAP was present. After 5 days, the TTR-A formed aggregates that did not enter the separation gel.