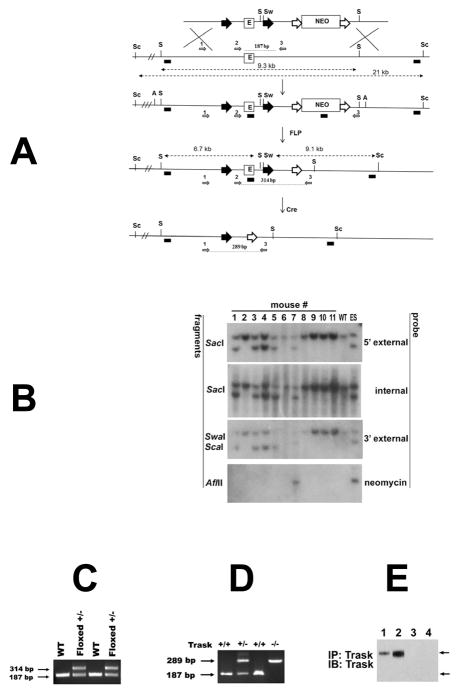
Figure 1. Targeting of the murine Trask locus.
(A) Diagrams of the targeted locus and construct used for the generation of Trask floxed and null mice. The loxp sites are shown as big black arrowheads that flank 1.4 kb fragment containing exon 1 (E) and core promoter sequences (not shown). Neomycin cassette (Neo) is flanked by FRT sites (big white arrows). The SacI (S), SwaI (Sw), Sca1 (Sc) and AflII (A) restriction site used for Southern analysis are indicated. The probes used for analysis include 5′ external, internal (exon1), 3′ external and Neo are depicted as small black boxes. The diagnostic SacI and Sca-1 SwaI fragments are shown with punctuated lines with double arrowheads. The removal of the Neo was achieved by crossing with actin-FLP mice, and generation of the null allele by crossing with EIIa-Cre transgenic mice. The PCR primers used for genotyping of floxed and null mice are depicted as small grey arrows and numbered 1, 2 and 3. (B) Southern analysis for germline transmission and Neo excision. Genomic DNA from the progeny of Trask chimeric mice and actin-FLP mice were digested with the indicated restriction enzymes and analyzed with 5′ extrenal, internal, 3′ external and Neo probes. DNA from wild type (WT) mouse and targeted ES clone were used as controls. Mice 1, 3, 4, and 5 are floxed +/− and used as founders for subsequent crossing. (C) Genotyping with primers 2 and 3 gives 187 bp product from the wt template and 314 bp product from the floxed allele. (D) PCR analysis of tail DNA with primers 1,2 and 3 were used to genotype Trask +/+, Trask +/− and Trask −/− mice. (E) The expression of Trask protein was assayed by immunoprecipitation and immunoblotting of lysates in mouse skin (1,3) and intestine (2,4) in Trask +/+ (1,2) and Trask −/− (3,4) mice. Only the 140 kD Trask is expressed in these mouse tissues.