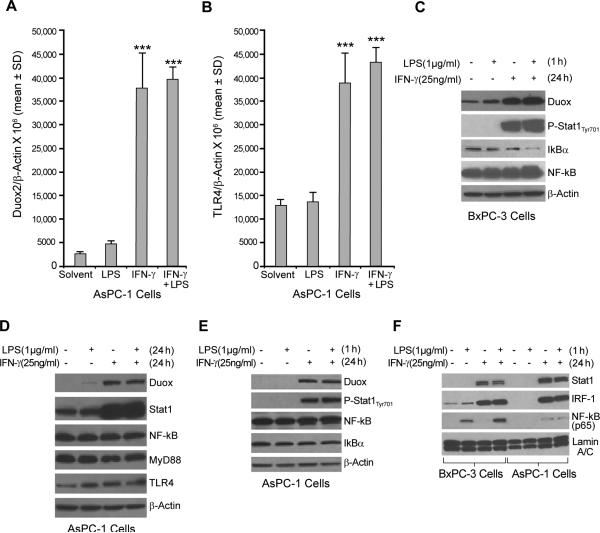
FIGURE 4.
LPS does not enhance IFN-γ–induced Duox2 expression in AsPC-1 human pancreatic cancer cells. A, Starved AsPC-1 cells were treated with IFN-γ (25 ng/ml) or LPS (1 μg/ml) for 24 h; 2 μg of total RNA was subjected to real time RT-PCR, and Duox2 expression relative to β-actin was determined; error bars represent standard deviations; data are from triplicate samples; *** = p < 0.001 versus AsPC-1 cells treated with solvent. B, TLR4 expression was determined using real-time RT-PCR with the same RNA as shown in panel A. Human TLR4-specific primers were used for PCR; *** = p < 0.001 versus AsPC-1 cells treated with solvent. C, LPS activated the NF-κB signaling pathway in BxPC-3 cells. BxPC-3 cells grown in serum-free medium were treated with solvent or IFN-γ (25 ng/ml) for 24 h and then incubated with or without LPS (1 μg/ml) for 1 h; 50 μg of whole cell extract was subjected to Western analysis. These experiments were performed in triplicate. D, LPS did not enhance IFN-γ–mediated Duox2 expression in AsPC-1 cells. Starved AsPC-1 cells were treated with IFN-γ (25 ng/ml) or LPS (1 μg/ml) for 24 h, and 50 μg of whole cell extract was subjected to Western analysis, using specific antibodies as indicated. The results are representative of triplicate experiments. E, LPS did not activate the NF-κB signaling pathway in AsPC-1 cells. AsPC-1 cells grown in serum-free medium were treated with solvent or IFN-γ (25 ng/ml) for 24 h and then incubated with or without LPS (1 μg/ml) for 1 h; 50 μg of whole cell extract was then subjected to Western analysis. The data shown are representative of three identical experiments. F, Effect of LPS on p65 nuclear accumulation in BxPC-3 and AsPC-1 cells exposed to IFN-γ. BxPC-3 and AsPC-1 cells were treated as described in panel A, and 20 μg of nuclear extract was subjected to Western analysis to determine Stat1, IRF-1, and p65 distribution in the nucleus. Lamin A/C was used as a loading control for nuclear protein. The data are representative of experiments performed in triplicate.