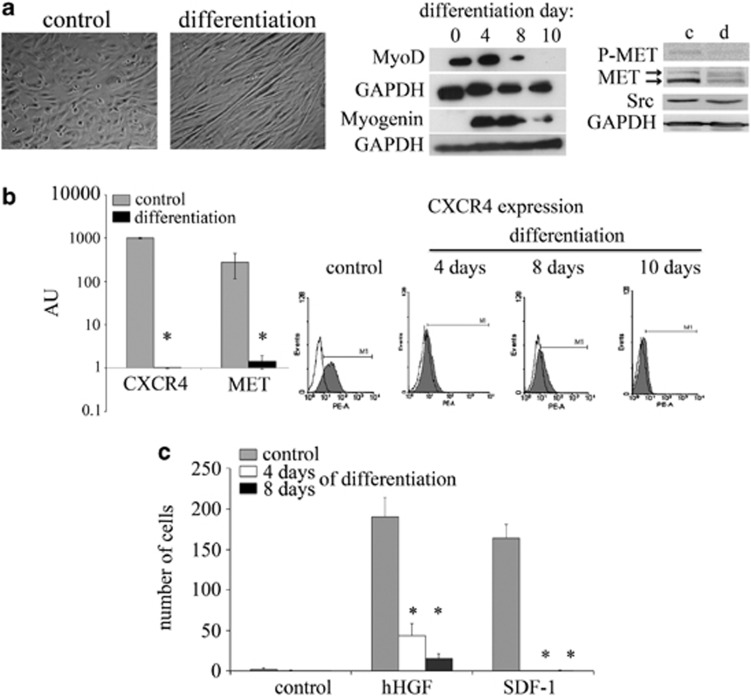
Figure 6.
Decreased expression and signaling of the MET and CXCR4 receptors in differentiated RMS cells. (a) The morphology of RH30 cells under differentiation inducing conditions. The differentiation process of RH30 cells was induced using low serum (2% HS) and TPA (100 nM). The cells were analyzed on days 4, 8 and 10. Beginning on day 4, morphological changes became apparent. The formation of characteristic elongated cellular structures was observed in cells cultured under differentiation conditions. The morphological changes were accompanied by the changes in the expression of early (MyoD) and late (myogenin) muscles differentiation markers. The expression of MyoD decreased to an undetectable level during the differentiation process. At the same time, the level of myogenin increased during the first 8 days (middle panel). Western blot analysis of phospho-MET and total MET showed significantly reduced signals for these proteins after 10 days of differentiation, in addition to a slight decrease of total Src protein. GAPDH was used as a loading control (right panel). The expression of the MET and CXCR4 receptors was evaluated in both undifferentiated and differentiated RH30 cell lines. (b) RT-PCR analysis of MET and CXCR4 receptor expression. The significant downregulation of the MET and CXCR4 receptors was observed at the mRNA level (left panel). Flow cytometry analysis of CXCR4 receptor expression in RH30 cells. The expression of the CXCR4 receptors decreased in a time-dependent manner and was completely gone after 10 days of differentiation (right panel). (c) Chemotactic assay of RH30 cells. The differentiated cells were subjected to a chemotaxis assay towards SDF-1 and human HGF gradients. A strong inhibition in the migration properties to both chemoattractants was observed. The experiment was repeated three times with similar results. Bar=50 μm; *P<0.05