Abstract
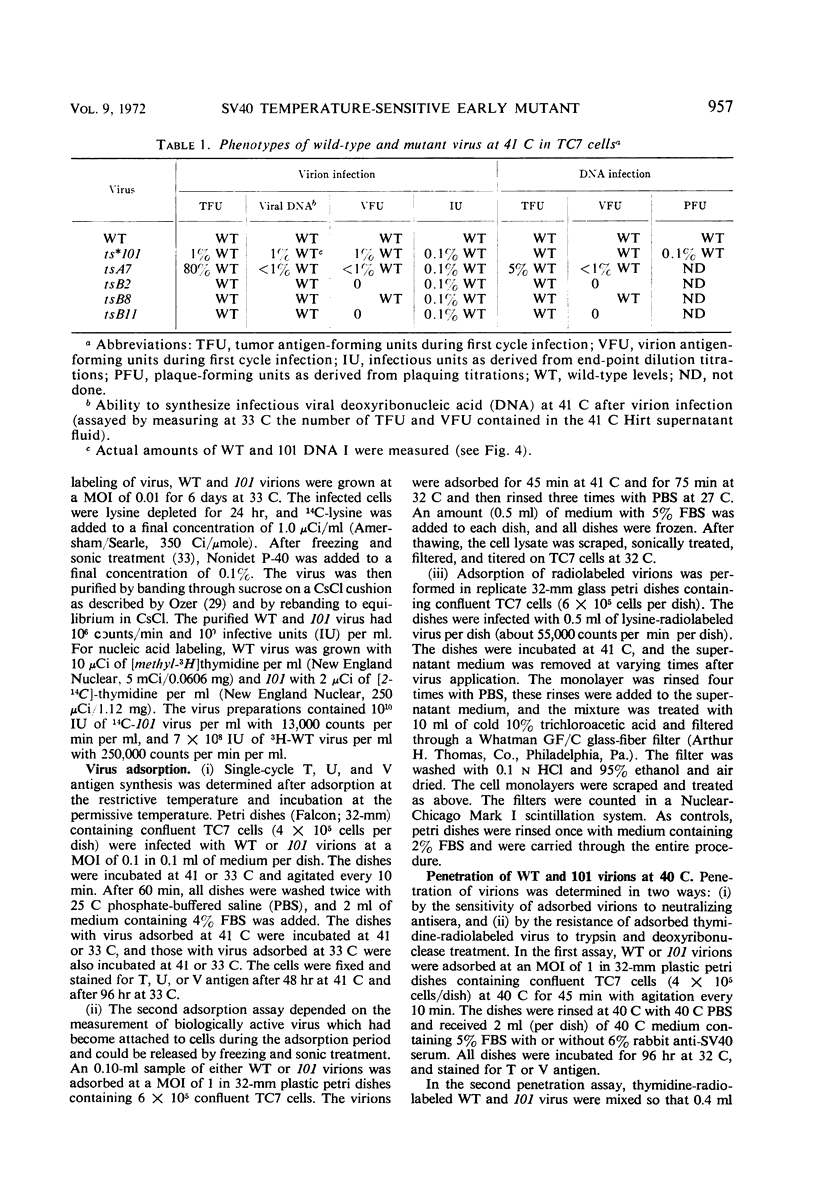
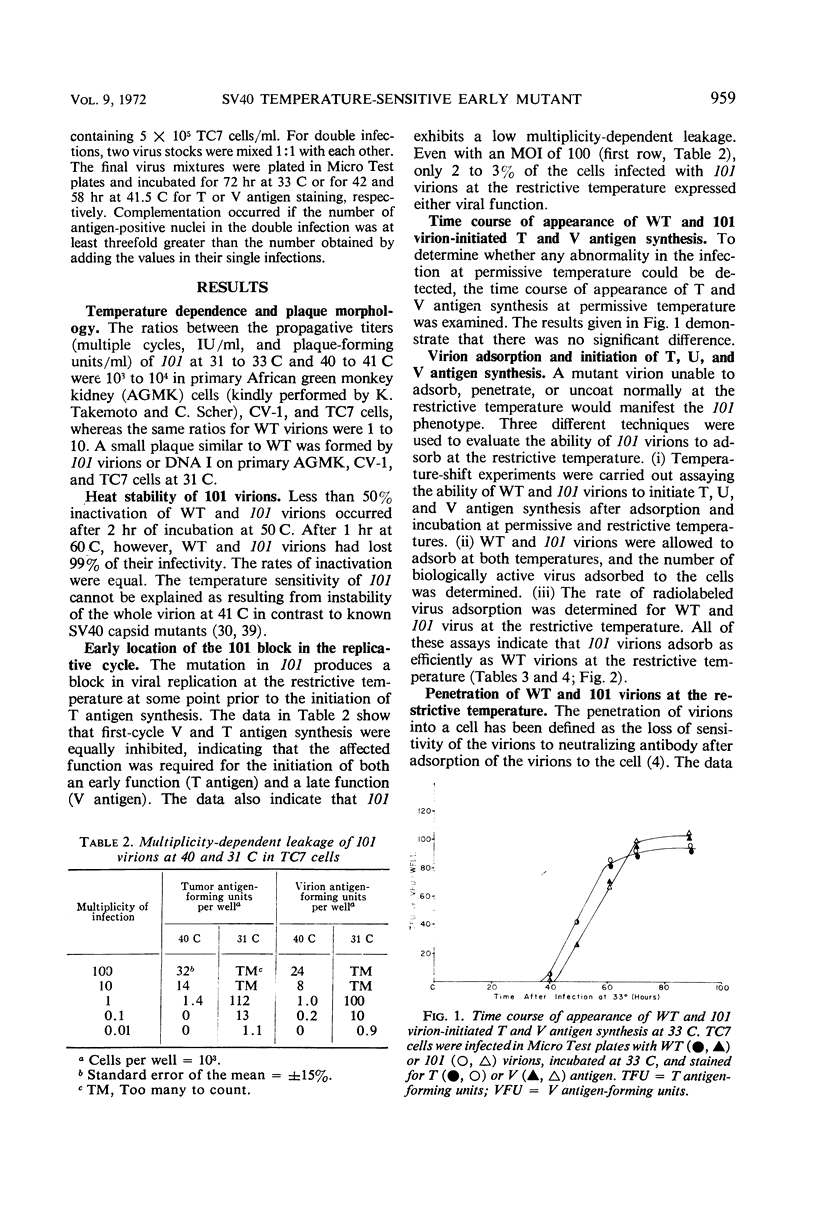
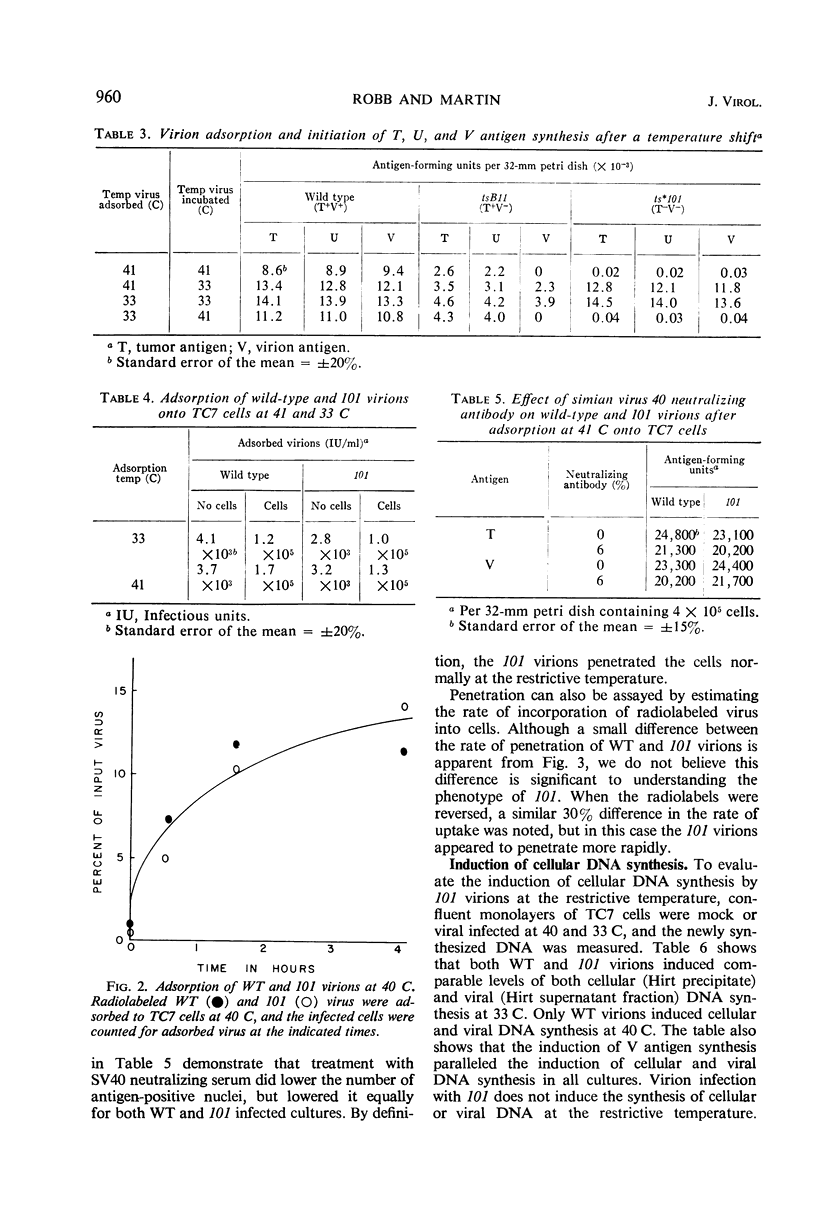
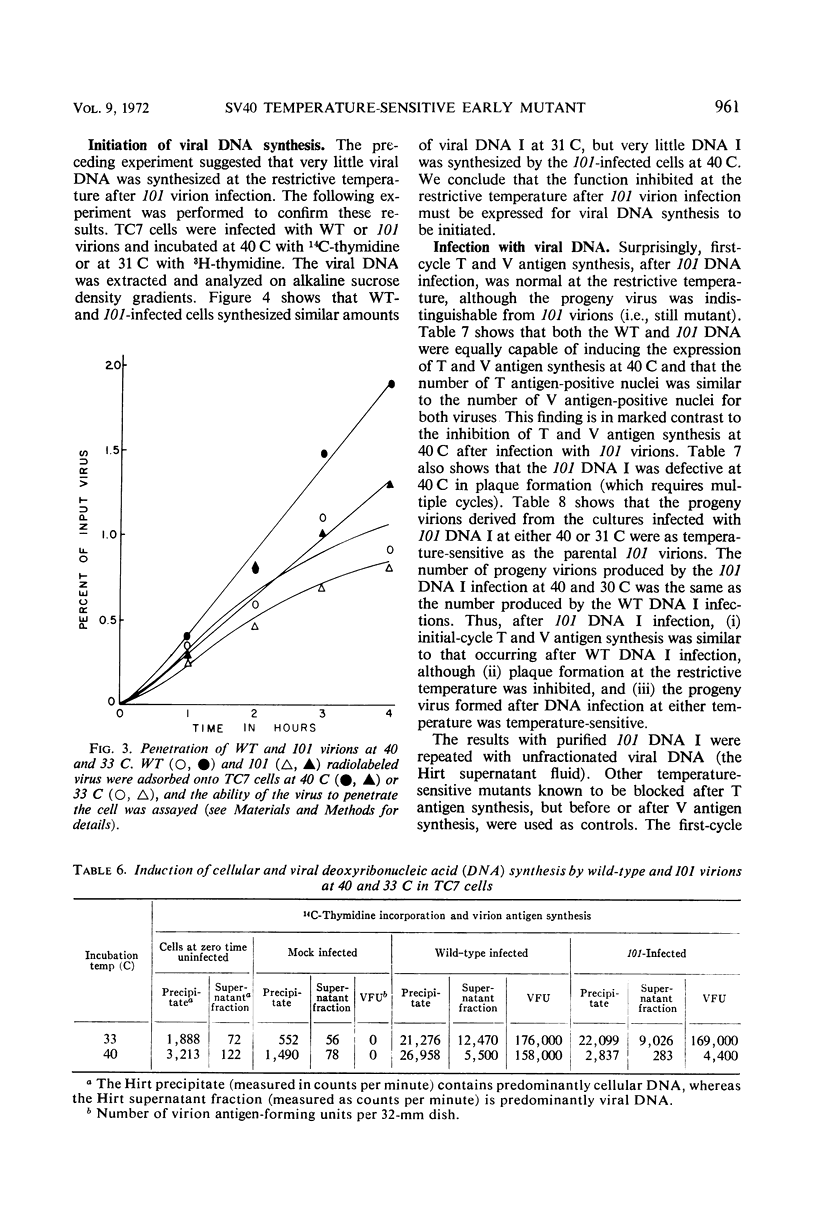
A temperature-sensitive mutant of simian virus 40 (SV40), ts*101, has been characterized during productive infection in monkey kidney cells. The mutant virion can adsorb to and penetrate the cell normally at the restrictive temperature, but cannot induce the synthesis of cellular deoxyribonucleic acid (DNA) nor initiate the synthesis of SV40-specific tumor, virion, or U antigens or viral DNA. First-cycle infection with purified ts*101 DNA is normal at the restrictive temperature, but the resulting progeny virions are still temperature-sensitive. The mutant neither complements nor inhibits other temperature-sensitive SV40 mutants or wild-type virions. The affected protein in the ts*101 mutant may be a regulatory structural protein, possibly a core protein, that is interacting with the viral DNA.
Full text
PDF

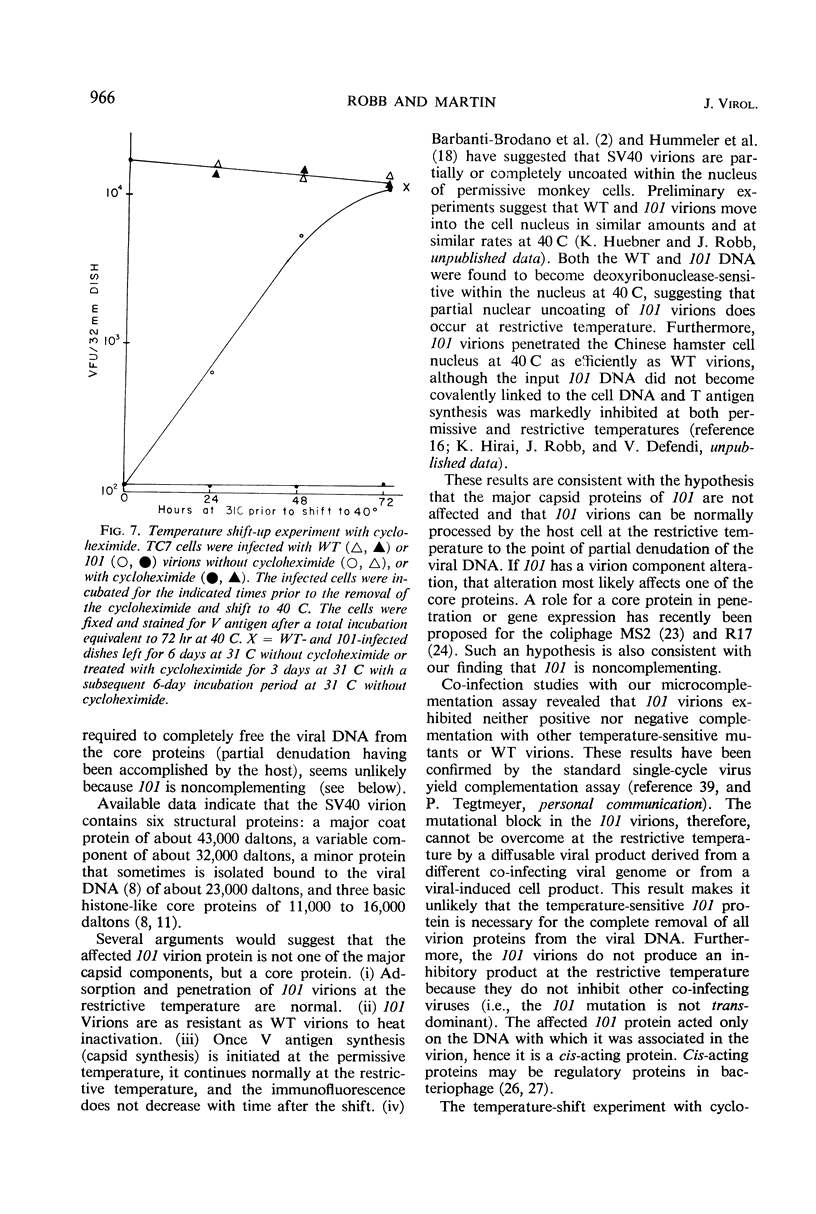


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A. Susceptibility of human cell strains to transformation by simian virus 40 and simian virus 40 deoxyribonucleic acid. J Virol. 1970 Oct;6(4):470–475. doi: 10.1128/jvi.6.4.470-475.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbanti-Brodano G., Swetly P., Koprowski H. Early events in the infection of permissive cells with simian virus 40: adsorption, penetration, and uncoating. J Virol. 1970 Jul;6(1):78–86. doi: 10.1128/jvi.6.1.78-86.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair D. G., Clewell D. B., Sheratt D. J., Helinski D. R. Strand-specific supercoiled DNA-protein relaxation complexes: comparison of the complexes of bacterial plasmids ColE1 and ColE2. Proc Natl Acad Sci U S A. 1971 Jan;68(1):210–214. doi: 10.1073/pnas.68.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARNELL J. E., Jr, SAWYER T. K. The basis for variation in susceptibility to poliovirus in HeLa cells. Virology. 1960 Aug;11:665–675. doi: 10.1016/0042-6822(60)90113-6. [DOI] [PubMed] [Google Scholar]
- De Crombrugghe B., Chen B., Gottesman M., Pastan I., Varmus H. E., Emmer M., Perlman R. L. Regulation of lac mRNA synthesis in a soluble cell-free system. Nat New Biol. 1971 Mar 10;230(10):37–40. doi: 10.1038/newbio230037a0. [DOI] [PubMed] [Google Scholar]
- Di Mayorca G., Callender J., Marin G., Giordano R. Temperature-sensitive mutants of polyoma virus. Virology. 1969 May;38(1):126–133. doi: 10.1016/0042-6822(69)90134-2. [DOI] [PubMed] [Google Scholar]
- Eckhart W. Complementation and transformation by temperature-sensitive mutants of polyoma virus. Virology. 1969 May;38(1):120–125. doi: 10.1016/0042-6822(69)90133-0. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Huang E. S., Pagano J. S. Structural polypeptides of simian virus 40. J Virol. 1971 May;7(5):635–641. doi: 10.1128/jvi.7.5.635-641.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M. Characterization of a temperature-sensitive mutant of polyoma virus. Virology. 1970 Mar;40(3):605–617. doi: 10.1016/0042-6822(70)90205-9. [DOI] [PubMed] [Google Scholar]
- Gershon D., Sachs L., Winocour E. The induction of cellular DNA synthesis by simian virus 40 in contact-inhibited and in x-irradiated cells. Proc Natl Acad Sci U S A. 1966 Sep;56(3):918–925. doi: 10.1073/pnas.56.3.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M., Marty L., Suarez F. Capsid proteins of Simian virus 40. Biochem Biophys Res Commun. 1970 Jul 13;40(1):97–102. doi: 10.1016/0006-291x(70)91051-x. [DOI] [PubMed] [Google Scholar]
- Green M. H., Miller H. I., Hendler S. Isolation of a polyoma-nucleoprotein complex from infected mouse-cell cultures. Proc Natl Acad Sci U S A. 1971 May;68(5):1032–1036. doi: 10.1073/pnas.68.5.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M., Dulbecco R. Induction of DNA synthesis by SV40. Proc Natl Acad Sci U S A. 1966 Aug;56(2):736–740. doi: 10.1073/pnas.56.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helinski D. R., Clewell D. B. Circular DNA. Annu Rev Biochem. 1971;40:899–942. doi: 10.1146/annurev.bi.40.070171.004343. [DOI] [PubMed] [Google Scholar]
- Herzberg M., Winocour E. Simian virus 40 deoxyribonucleic acid transcription in vitro: binding and transcription patterns with a mammalian ribonucleic acid polymerase. J Virol. 1970 Nov;6(5):667–676. doi: 10.1128/jvi.6.5.667-676.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Lehman J., Defendi V. Integration of simian virus 40 deoxyribonucleic acid into the deoxyribonucleic acid of primary infected Chinese hamster cells. J Virol. 1971 Nov;8(5):708–715. doi: 10.1128/jvi.8.5.708-715.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hummeler K., Tomassini N., Sokol F. Morphological aspects of the uptake of simian virus 40 by permissive cells. J Virol. 1970 Jul;6(1):87–93. doi: 10.1128/jvi.6.1.87-93.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENSEN F. C., GIRARDI A. J., GILDEN R. V., KOPROWSKI H. INFECTION OF HUMAN AND SIMIAN TISSUE CULTURES WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1964 Jul;52:53–59. doi: 10.1073/pnas.52.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Tokuno S., Nakajima K., Trkula D., Dubbs D. R. Temperature-sensitive simian virus 40 mutant defective in a late function. J Virol. 1970 Sep;6(3):286–294. doi: 10.1128/jvi.6.3.286-294.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M., Nathans D. Fate of maturation protein during infection by coliphage MS2. Nat New Biol. 1971 Sep 15;234(50):209–211. doi: 10.1038/newbio234209a0. [DOI] [PubMed] [Google Scholar]
- Krahn P. M., O'Callaghan R. J., Paranchych W. Stages in phage R17 infection. VI. Injection of A protein and RNA into the host cell. Virology. 1972 Mar;47(3):628–637. doi: 10.1016/0042-6822(72)90552-1. [DOI] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Levin M. J., Wiese W. H., Crumpacker C. S., Henry P. H. A nondefective (competent) adenovirus-SV40 hybrid isolated from the AD.2-SV40 hybrid population. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1128–1135. doi: 10.1073/pnas.63.4.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl G. Bacteriophage P2: replication of the chromosome requires a protein which acts only on the genome that coded for it. Virology. 1970 Oct;42(2):522–533. doi: 10.1016/0042-6822(70)90295-3. [DOI] [PubMed] [Google Scholar]
- Lindahl G., Hirota Y., Jacob F. On the process of cellular division in Escherichia coli: replication of the bacterial chromosome under control of prophage P2. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2407–2411. doi: 10.1073/pnas.68.10.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Ozer H. L. Synthesis and assembly of simian virus 40. I. Differential synthesis of intact virions and empty shells. J Virol. 1972 Jan;9(1):41–51. doi: 10.1128/jvi.9.1.41-51.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer H. L., Takemoto K. K. Site of host restriction of simian virus 40 mutants in an established African green monkey kidney cell line. J Virol. 1969 Oct;4(4):408–415. doi: 10.1128/jvi.4.4.408-415.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUCK T. T., CIECIURA S. J., ROBINSON A. Genetics of somatic mammalian cells. III. Long-term cultivation of euploid cells from human and animal subjects. J Exp Med. 1958 Dec 1;108(6):945–956. doi: 10.1084/jem.108.6.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb J. A., Martin R. G. Genetic analysis of simian virus 40. I. Description of microtitration and replica-plating techniques for virus. Virology. 1970 Aug;41(4):751–760. doi: 10.1016/0042-6822(70)90439-3. [DOI] [PubMed] [Google Scholar]
- Robb J. A., Smith H. S., Scher C. D. Genetic analysis of simian virus 40. IV. Inhibited transformation of Balb-3T3 cells by a temperature-sensitive early mutant. J Virol. 1972 Jun;9(6):969–972. doi: 10.1128/jvi.9.6.969-972.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb J. A., Tegtmeyer P., Martin R. G., Kit S. Proposal for a uniform nomenclature for simian virus 40 mutants. J Virol. 1972 Mar;9(3):562–563. doi: 10.1128/jvi.9.3.562-563.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. D., Barnett L., Brenner S., Russell R. L. More mutant tyrosine transfer ribonucleic acids. J Mol Biol. 1970 Nov 28;54(1):1–14. doi: 10.1016/0022-2836(70)90442-0. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Kirschstein R. L., Habel K. Mutants of simian virus 40 differing in plaque size, oncogenicity, and heat sensitivity. J Bacteriol. 1966 Oct;92(4):990–994. doi: 10.1128/jb.92.4.990-994.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Dohan C., Jr, Reznikoff C. Inactivating and mutagenic effects of nitrosoguanidine on simian virus 40. Proc Natl Acad Sci U S A. 1970 Jul;66(3):745–752. doi: 10.1073/pnas.66.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Ozer H. L. Temperature-sensitive mutants of simian virus 40: infection of permissive cells. J Virol. 1971 Oct;8(4):516–524. doi: 10.1128/jvi.8.4.516-524.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Aaronson S. A., Rands E. Rapid detection of mycoplasma-infected cell cultures. Exp Cell Res. 1971 Mar;65(1):256–257. doi: 10.1016/s0014-4827(71)80077-0. [DOI] [PubMed] [Google Scholar]
- Travers A. A. Positive control of transcription by a bacteriophage sigma factor. Nature. 1970 Mar 14;225(5237):1009–1012. doi: 10.1038/2251009a0. [DOI] [PubMed] [Google Scholar]


