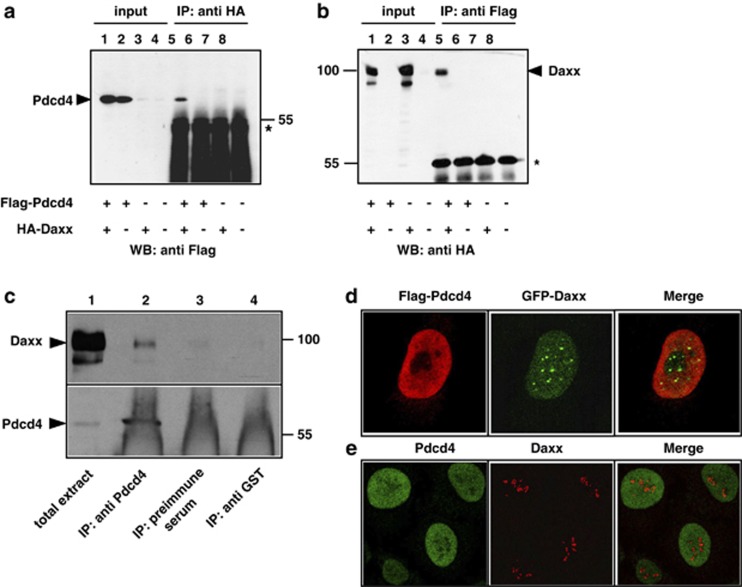
Figure 1.
Co-immunoprecipitation and subnuclear localization of Pdcd4 and Daxx. (a, b) QT6 cells were transfected with the indicated combinations of plasmids encoding Flag-Pdcd4 and HA-Daxx. Cells were lysed after 24 h and protein extracts were immunoprecipitated with anti-Flag or anti-HA antibodies, as indicated above the panels. Proteins were analyzed by SDS–PAGE and western blotting, using the antibodies indicated below the panels. Analyses of the crude protein extracts (input) demonstrate comparable expression levels of the proteins in the different samples. HA-Daxx and Flag-Pdcd4 are marked by arrowheads. The asterisks mark the immunoglobulin heavy chains of the HA and Flag antibodies. (c) Protein extracts of HeLa cells were immunoprecipitated with an antiserum against endogenous human Pdcd4 (lane 2). Controls were performed with preimmune serum from the same animal (lane 3) or with an antiserum against tubulin (lane 4). Total cell extract (lane 1) and precipitated proteins were analyzed by SDS–PAGE, followed by western blotting using an antiserum against Daxx (upper panel) or Pdcd4 (bottom panel). Daxx and Pdcd4 are marked by black arrowheads. The strong diffuse staining in lanes 2–4 at the bottom of the lower panel is due to the immunoglobulins from the antiserum used for immunoprecipitation. (d) HeLa cells were transfected with expression vectors for Flag-Pdcd4 and GFP-Daxx. After 24 h, cells were fixed and Flag-Pdcd4 was stained with anti-Flag and tetramethyl rhodamine iso-thiocyanate-conjugated secondary antibody (red). GFP-Daxx was detected using intrinsic GFP fluorescence (green). (e) Non-transfected HeLa cells were stained with antiserum against endogenous Pdcd4 (green) and endogenous Daxx (red).