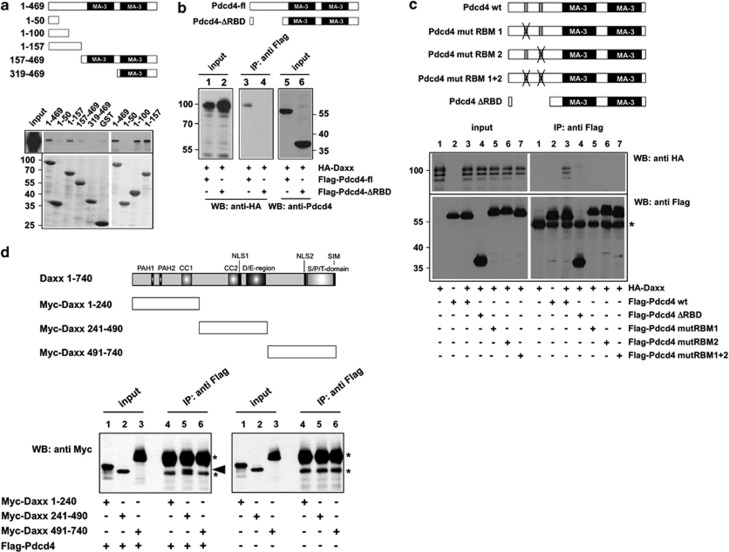
Figure 2.
Mapping of the Daxx and Pdcd4 binding sites. (a) GST-pull down experiments were performed with the indicated GST and GST-Pdcd4 fusion proteins, which were incubated with lysates of QT6 cells transfected with an expression vector for HA-Daxx. Bound proteins were analyzed by SDS–PAGE, followed by western blotting with antibodies against the HA-tag. Total protein extract of the transfected cells was used as control (input). Coomassie blue staining of GST proteins used in the pull-down experiments demonstrates comparable protein amounts (lower panels). (b, c) QT6 cells were transfected with the indicated combinations of plasmids encoding full-length Flag-Pdcd4, N terminally truncated or point-mutated versions of Flag-Pdcd4, and HA-Daxx. Cells were lysed after 24 h and protein extracts were immunoprecipitated with anti-Flag antibodies, followed by SDS–PAGE and western blotting, using the indicated antibodies. Analyses of the crude protein extracts (input lanes) demonstrate comparable expression levels of the proteins in the different samples. The asterisk in panel c marks the immunoglobulin heavy chain of the Flag antibody. The Pdcd4 constructs used are shown schematically at the top of panels a–c. (d) QT6 cells were transfected with the indicated combinations of plasmids encoding full-length Flag-Pdcd4 and Myc-tagged Daxx proteins. Cells were lysed after 24 h and protein extracts were immunoprecipitated with anti-Flag antibodies, followed by SDS–PAGE and western blotting with anti-Myc antibodies. Aliquots of the crude protein extracts (input lanes) were analyzed to demonstrate the expression levels and the sizes of the Daxx constructs. The Myc-Daxx (241–490) protein is marked by an arrowhead. Asterisks mark the immunoglobulin heavy chain of the Flag antibody and a nonspecific protein band present in all immunoprecipitates. The Daxx constructs used are shown schematically at the top.