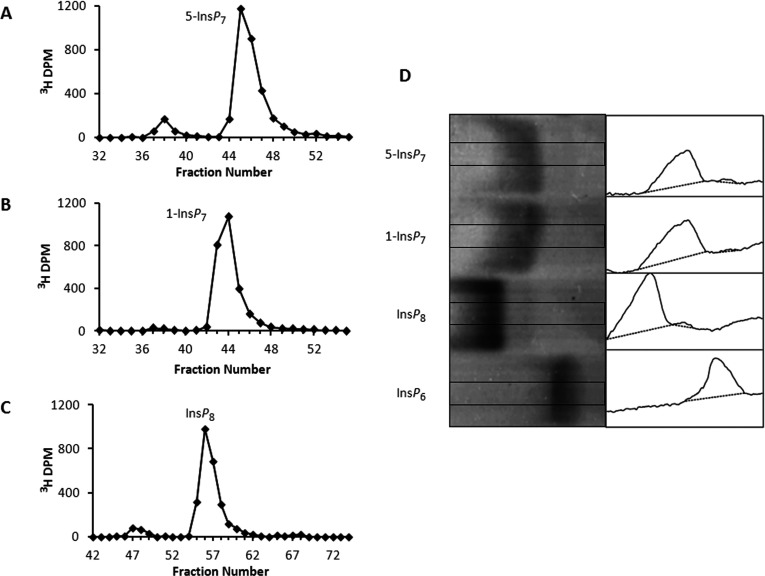
Figure 2. Purity of PP-InsP preparations.
HPLC (see the Materials and methods section) of 5-[3H]InsP7 (A), 1-[3H]InsP7 (B) and [3H]InsP8 (C). The purities of 5-[3H]InsP7, 1-[3H]InsP7 and [3H]InsP8 were 91, 98 and 92%, respectively. (D) Toluidine Blue-stained PAGE analysis (16 cm gel) of purity of non-radiolabelled PP-InsP preparations (20 nmol/lane). The image was enhanced in ImageJ [54] by increasing contrast and smoothing. Purity was quantified by densitometric analysis (right-hand panels) of the central portion of each lane as indicated by the rectangles in each of the left-hand panels using ImageJ [54]. Commercial InsP6 (not further purified) served as a control. Purity was as follows: 5-InsP7, 96%; 1-InsP7, 98%; and InsP8, 97%.