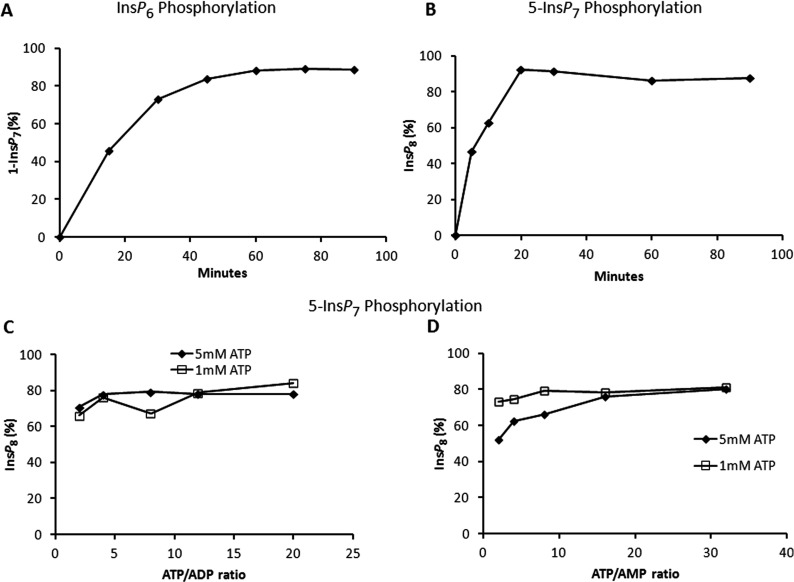
Figure 7. Effect of ATP/ADP and AMP upon the equilibrium condition for PPIP5K2KD.
(A) and (B) show a time course of InsP6 and 5-InsP7 phosphorylation, respectively. In (A), 20 μg/ml enzyme was incubated with reaction buffer (see the Materials and methods section) containing 15 μM InsP6, 5 mM ATP and 0.5 mM ADP. In (B), 0.33 μg/ml enzyme was incubated with reaction buffer (see the Materials and methods section) containing 1 μM 5-InsP7 and 5 mM ATP. Assays were quenched and neutralized and analysed by Partisphere SAX HPLC as described in the Materials and methods section. (C) and (D) show the equilibrium point for the phosphorylation of 5-InsP7 to InsP8 in which 0.33 μg/ml enzyme was incubated as described in the Materials and methods section under initial conditions of 1 μM 5-InsP7 and either 1 (squares) or 5 (diamonds) mM ATP; either the ATP/ADP ratio (C) or the ATP/AMP ratio (D) was varied as indicated. Assays were conducted for 60 min. The reactions were then quenched and neutralized and analysed by Partisphere SAX HPLC as described in the Materials and methods section.