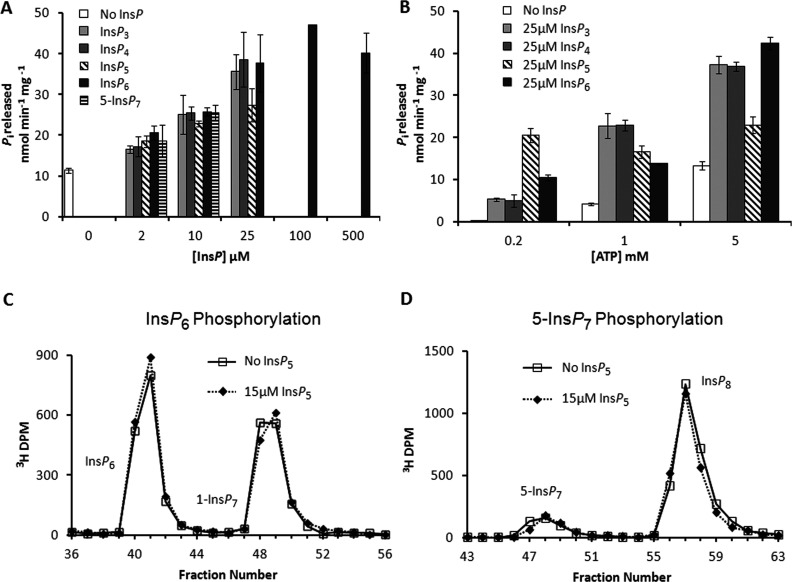
Figure 8. ATPase activity of PPIP5K2KD.
(A) and (B) Orthophosphate released from the hydrolysis of ATP was measured using a Malachite Green detection method [55]. The 27–270 μg/ml PPIP5K2KD was incubated at 37°C for 120 min in 50 μl reactions containing 20 mM Tris/HCl, pH 7.5, 10 mM ATP (unless otherwise indicated), 100 mM KCl, 0.1 mM EDTA and 5 mM MgCl2. (A) Stimulation of PPIP5K2KD ATPase activity by InsP3, InsP4, InsP5, InsP6 and 5-InsP7 (concentrations as indicated, [ATP]=5 mM). (B) Effect of ATP concentration (0.2, 1 or 5 mM) on basal (absence of InsP) and InsP-stimulated (25 μM) ATPase activity. Results are presented as the means±S.D. (n=3). (C) and (D) provide representative HPLC data indicating that 15 μM InsP5 (dotted lines) does not inhibit phosphorylation of either InsP6 or 5- InsP7, respectively. In (C), 20 μg/ml PPIP5K2KD was incubated for 15 min with reaction buffer (see the Materials and methods section) containing 15 μM [3H]InsP6, 5 mM ATP and 0.5 mM ADP. In (D), 0.41 μg/ml PPIP5K2KD was incubated for 5 min with reaction buffer (see the Materials and methods section) containing 1 μM 5-[3H]InsP7, 5 mM ATP and 0.5 mM ADP. The reactions were quenched and neutralized and analysed by Partisphere SAX HPLC as described in the Materials and methods section.