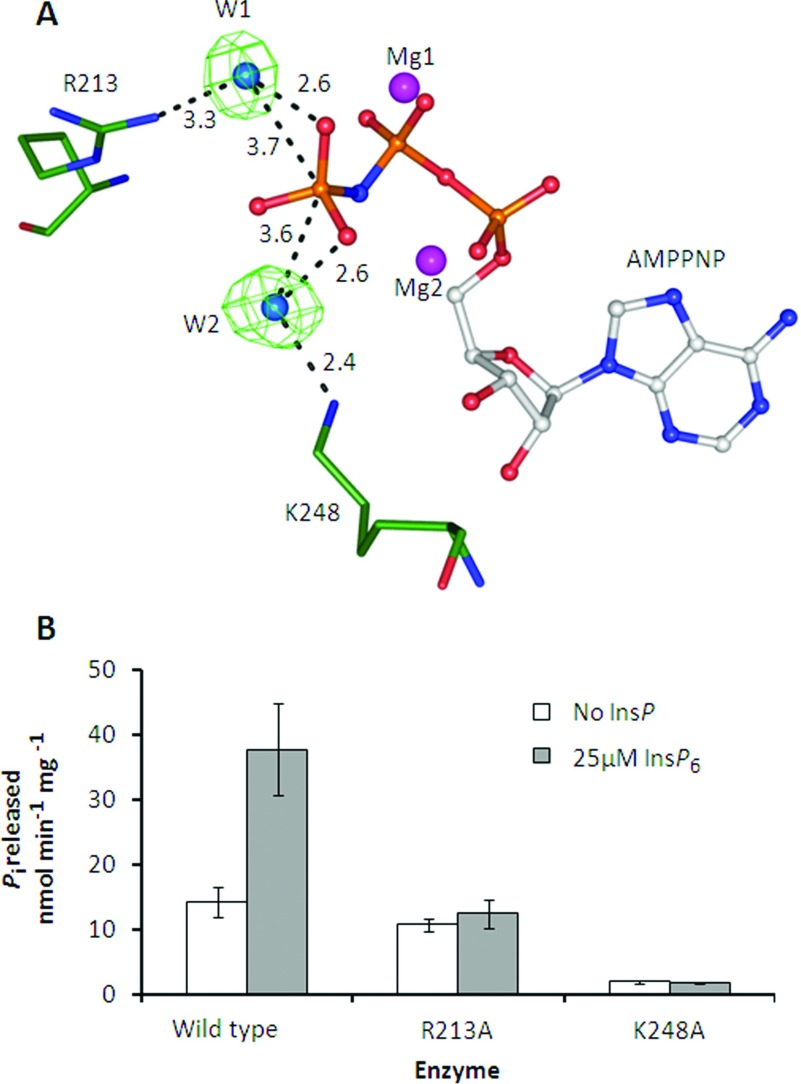
Figure 9. The effects of R213A and K248A mutations upon ATPase activity of PPIP5K2KD.
(A) Structure of a portion of the wild-type PPIP5K2KD catalytic centre [20] showing water molecules bridging the interaction between the ATP analogue AMPPNP (adenosine 5′-[β,γ-imido]triphosphate) and R213 and K248. (B) The effect of single-site mutants of PPIP5K2KD on basal and InsP6-stimulated (25 μM) ATPase activity. ATPase activity was measured as in Figure 8.