Appendix Figure A1.
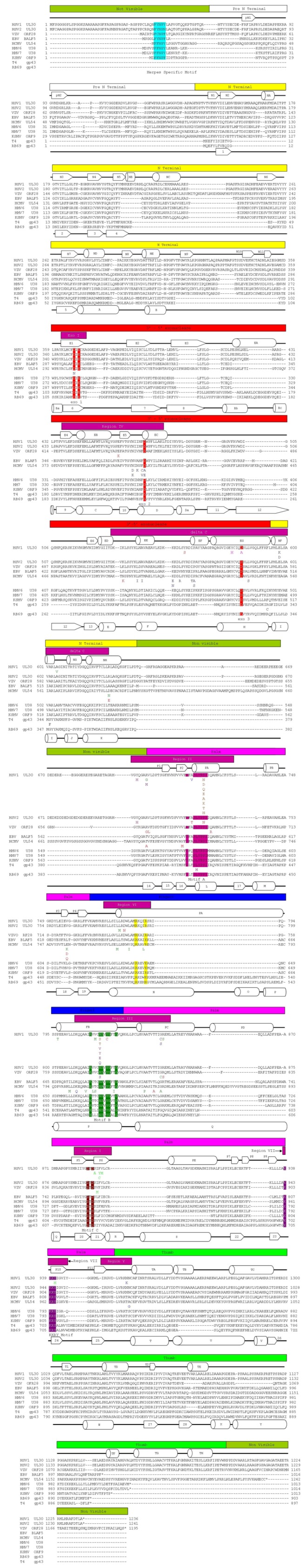
Protein sequence alignment of herpesvirus and bacteriophage polymerases based on structural data. Herpesvirus and bacteriophage polymerase were aligned individually using the muscle algorithm within Geneious [24,25]. Then the bacteriophage and herpesvirus sequences were aligned using an alignment based on a structural alignment of HSV1 UL30 (2GV9) and RB69 gp43 (1IH7) produced by RAPIDO [26]. Blocks above sequence highlight structural domains of polymerase. Regions unresolved in the HSV1 structural model are shown in green. The pre N terminal domain is in white. The N terminal domain is in yellow. The 3'–5' exonuclease domain is in red. The palm domain is in pink. The fingers domain is in blue and thumb domain is in green. The known conserved regions are shown in magenta blocks above sequence [27,28,29,30,31]. Secondary structural elements of HSV1 UL30 are indicated. Elements are number according scheme provided in Liu et al. (2006). Secondary Structural elements of RB69 gp43 are indicated. Elements are numbered according scheme provided in Wang et al. (1997). Structural motifs involved in polymerase and exonuclease activity are highlighted in sequence. Herpes virus specific motif is in blue. Exonuclease conserved residues are in red. Motif A is in magenta. N helix residues are in yellow. Motif B is in green. Motif C is in brown. KKRY motif is in purple. Mutations that have been associated with resistance to anti-herpetic drugs are shown below the corresponding residue [32,33]. Resistance conferring mutations are colored using the following scheme: Red: PyrophosphateR (Resistant), Blue: NucleotideR, Green: PyrophosphateR and NucleotideR. Purple: PyrophosphateHS (Hypersensitive). Pink: PyrophosphateR but NucleotideHS, Brown: NucleotideR but PyrophosphateHS.
