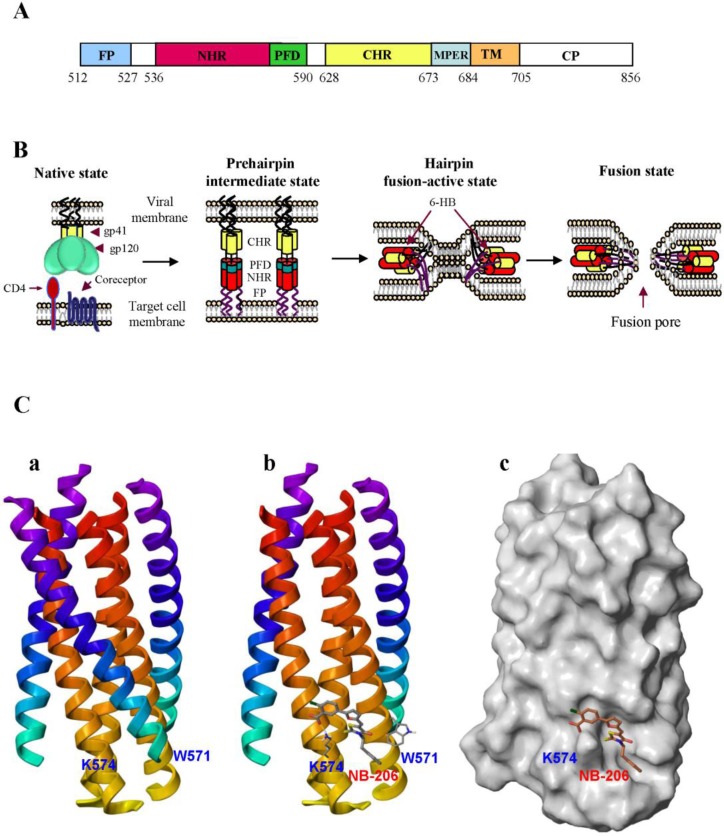
Figure 1.
Model of HIV-1 gp41-mediated membrane fusion and interaction of the HIV-1 entry inhibitor with the pocket in gp41. (A) Schematic view of the HIV-1HXB2 gp41 molecule. FP, fusion peptide; NHR, N-terminal heptad repeat; PFD, pocket-forming domain; CHR, C-terminal heptad repeat; MPER, membrane-proximal external region; TM, transmembrane domain; and CP, cytoplasmic domain. (B) Model of HIV-1 gp41-mediated membrane fusion. Fusion of the HIV-1 envelope and target cell membrane is initiated by binding of the viral Env surface subunit gp120 to the cellular CD4 and then to a coreceptor (CCR5 or CXCR4) on the target cell. The Env transmembrane subunit gp41 changes conformation by inserting the FP into the target cell membrane and forming 6-HB between the viral gp41 NHR and CHR regions, bringing the viral and target cell membranes into close proximity for fusion (C) The crystal structure of the gp41 6-HB and docking of NB-206 in the gp41 hydrophobic pocket cavity. (a) Side view of the gp41 6-HB core structure formed by the N-peptide, N36, and C-peptide, C34. (b) Stereo view of NB-206 docked in the hydrophobic pocket showing the possible interactions with the neighboring hydrophobic and charged residue K574. (c) Surface representation of the gp41 core (with one C-peptide removed) with bound ligand NB-206, which docks inside the cavity with the negatively charged COOH group pointing towards the positively charged side chain of K574.