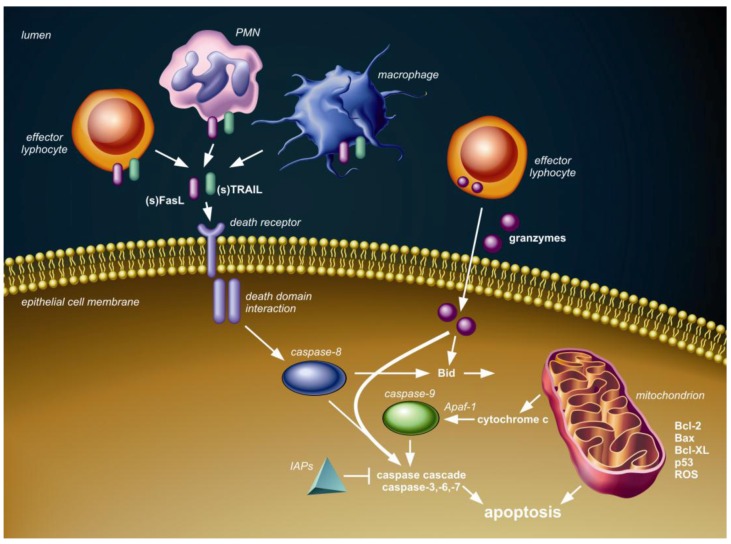
Figure 1.
Schematic overview of three pathways of caspase-dependent apoptosis. First, the death receptor (extrinsic) pathway is activated upon tumor necrosis factor (TNF) death receptor family ligation by membrane-bound or soluble ligands, such as Fas ligand (FasL) and TNF-related apoptosis-inducing ligand (TRAIL), presented or secreted by local immune cells, including effector lymphocytes, neutrophils (PMN) and/or macrophages. Intracellular adaptor protein interactions through death domain modules follow the death receptor ligation and subsequently lead to activation of initiator caspase-8 and the downstream caspase cascade resulting in apoptosis. The inhibitor of apoptosis proteins (IAPs) can block several caspases, thereby inhibiting cell death. Second, granzymes delivered into the cytosol by effector lymphocytes can interact with several caspases and Bid to induce apoptosis. Third, members of the Bcl-2 family, including Bcl-2, Bax and Bcl-XL and p53, regulate cytochrome c release from the mitochondria (intrinsic pathway) in response to stimuli, such as DNA damage, infection and formation of reactive oxygen species (ROS). Cytochrome c in the cytosol assembles with apoptotic peptidase activating factor 1 (Apaf 1) to activate initiator caspase-9 with subsequent activation of the caspase-cascade and apoptosis. The mitochondrial and death receptor pathway can interact through BH3-interacting domain death agonist (Bid).