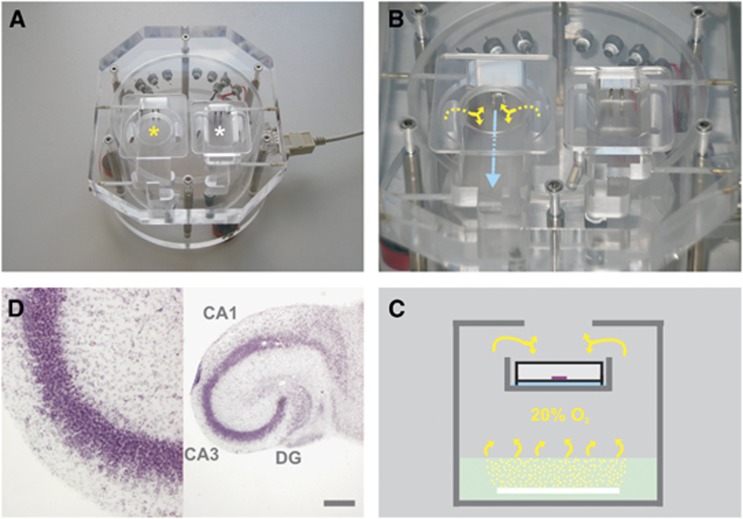
Figure 1.
Illustration of recording conditions and organotypic hippocampal slice cultures. (A) The custom-built acrylic glass unit contains two recording chambers that are used for maintenance of slice cultures on intact Biopore membrane inserts (left chamber, yellow asterisk) or acute brain slices (right chamber, white asterisk). (B) The recording solution (blue arrow) is supplied by an external peristaltic pump (not shown); the gas mixture (yellow arrows) is constantly exchanged. (C) The simplified scheme illustrates the functional design of the unit. The bottom part contains distilled water (mint rectangle) that is permanently warmed to 34±1°C. The gas mixture (20% O2) is bubbled into the distilled water via a looped perforated plastic tube (white bar) for warming and humidification and guided (yellow arrows) to the intermediate floor. On the intermediate floor, the warmed and gas-saturated recording solution (blue bar) flows underneath the intact Biopore membrane insert (white rectangle with black lines; open from above) carrying slice cultures (purple bar). For recordings of local field potential (LFP) and partial oxygen pressure (pO2), microelectrodes are positioned in slice cultures, through an opening in the cover plate and by using micromanipulators (not shown). (D) Morphologic characteristics of organotypic hippocampal slice cultures. The slice was maintained in culture for 12 days, then fixed, sectioned, and stained with cresyl violet. Note the ‘organotypic' preservation of hippocampal areas, CA1, CA3, and dentate gyrus (DG), including cell layers and functional connections (right image). Recordings of LFP and pO2 depth profiles were conducted in stratum pyramidale in area CA3 (purple cell layer, left image). The scale bar denotes 500 μm.