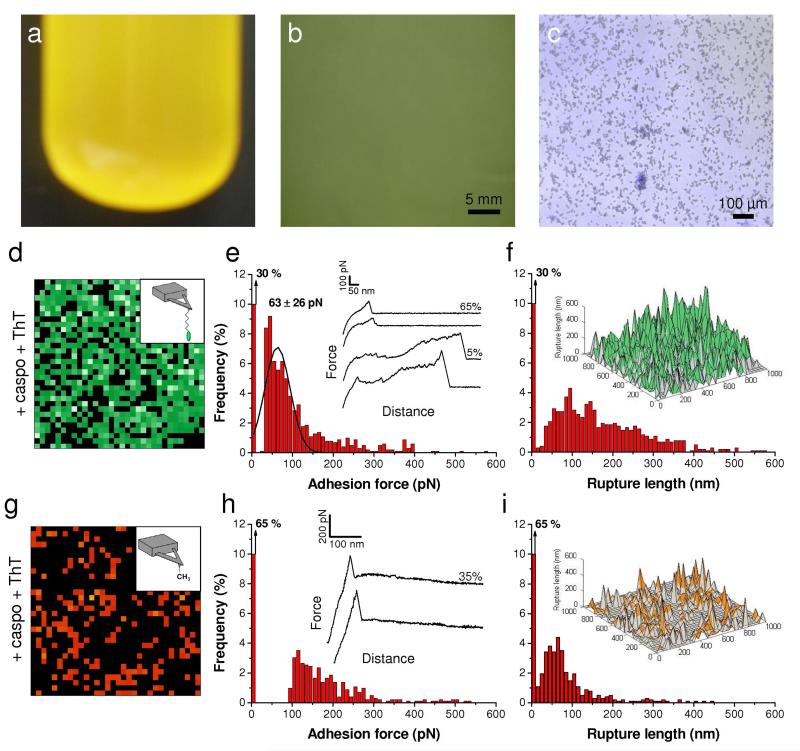
Fig. 8. Cell aggregation, Als1 unfolding and cell surface hydrophobicity require amyloid interactions.
(a, b, c) Micrograph (a), optical microscopy (b) and overlaid optical and fluorescence microscopy (c) images of C. albicans WT cells grown in the presence of 50 ng ml−1 caspofungin and 100 μM thioflavin T (ThT) documenting the inhibition of cell aggregation by ThT. (d, g) Adhesion force maps (1 μm × 1 μm, color scale: 350 pN) recorded in buffer with an adhesion peptide-tip (d) or a hydrophobic-tip (g) on C. albicans WT cells grown in the presence of caspofungin (50 ng ml−1), harvested and further treated with 100 μM ThT. (e, h) Corresponding adhesion force histograms (n = 1024) together with representative force curves. (f, i) Histograms of rupture distances (n = 1024), and 3-D reconstructed polymer maps (false colors, adhesion forces in green for peptide-tip and orange for hydrophobic-tip).