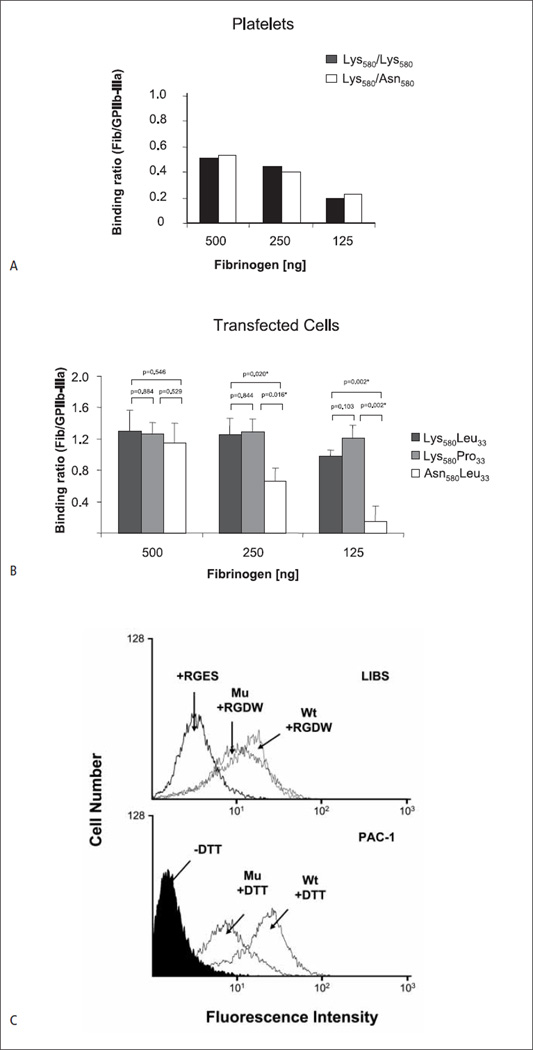
Figure 5. Effects of the Lys580Asn substitution on αIIbβ3 function.
A) Adhesion of homozygous Seca-negative (Lys580/Lys580) and heterozygous Seca-positive (Lys580/Asn580) platelets onto different concentrations of immobilised fibrinogen. Both platelets are typed as homozygous for HPA-1a (Leu33/Leu33). Fluorescence labelled platelets was allowed to adhere to microtitre wells coated either with BSA, mab Gi5 or fibrinogen. After washings, bound cells were measured on a fluorescence microtiter reader. Binding was calculated as ratio between the cells bound to fibrinogen and to mab Gi5 (n = 2). B) Adhesion of CHO cells expressing Leu33Lys580 (wild-type; HPA-1a), Pro33Lys580 (mutant; HPA-1b) and Leu33Asn580 (Seca) β3 isoforms on immobilised fibrinogen. Cell adhesion was performed as described above (n=5). C) Flow cytometry analysis of stably transfected cells expressing wild-type and mutant β3. The expression of αIIbβ3 was comparable between the two cell lines (mean fluorescence intensity (MFI; mean ±SD) for Asn580 = 557±89 and MFI for Lys580 = 702 ±74). Upper panel shows the binding of LIBS antibody after treatment with RGDW or RGEW peptide (as control); lower panel the binding of PAC-1 antibody with and without activation by DTT. Bound antibody was detected with fluorescein-labelled secondary antibody.