Figure 12.
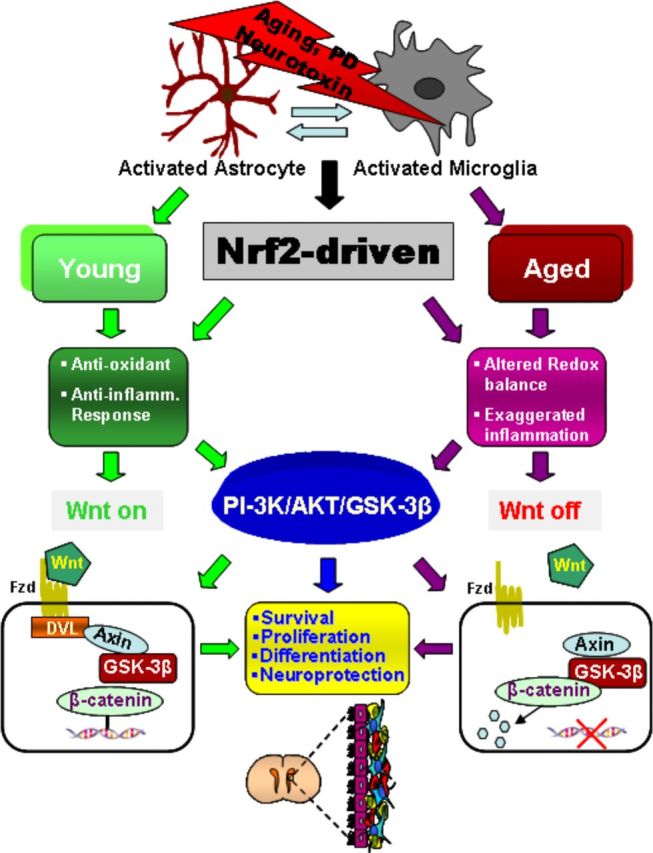
Simplified scheme summarizing the effect of aging and MPTP on Nrf2-ARE axis dysfunction-mediated Wnt/β-catenin signaling dysregulation in NPCs associated with neurogenic impairment. In young mice, a regulatory circuit linking microglial activation and proinflammatory cytokine to Nrf2-ARE protective pathway in SVZ, provides an efficient self-adaptive mechanism against inflammatory/neurotoxin-induced oxidative stress. In addition to governing the redox balance within the SVZ niche, the Nrf2-induced Hmox target gene may simultaneously protect astrocytes, thereby upregulating the expression of vital Wnt signaling elements that switch on key components required for maintaining SVZ cells in a proliferative state, for promoting differentiation, and/or for exerting neuroprotective effects. Cross talk between two pivotal pathways, the PI3-K/Akt/GSK-3β and Wnt/β-catenin signaling cascades, appears to finely control the transcriptional activator, β-catenin, which in turn represents a point of convergence to direct proliferation/differentiation/survival in the SVZ stem niche. Importantly, SVZ “rejuvenation” may have beneficial consequences for DAergic neuroprotection, and vice versa. Astrocytes (blue), neuroblasts (red), transit-amplifying cells (yellow), and ependymal cells (purple) in SVZ niche are schematically illustrated.
