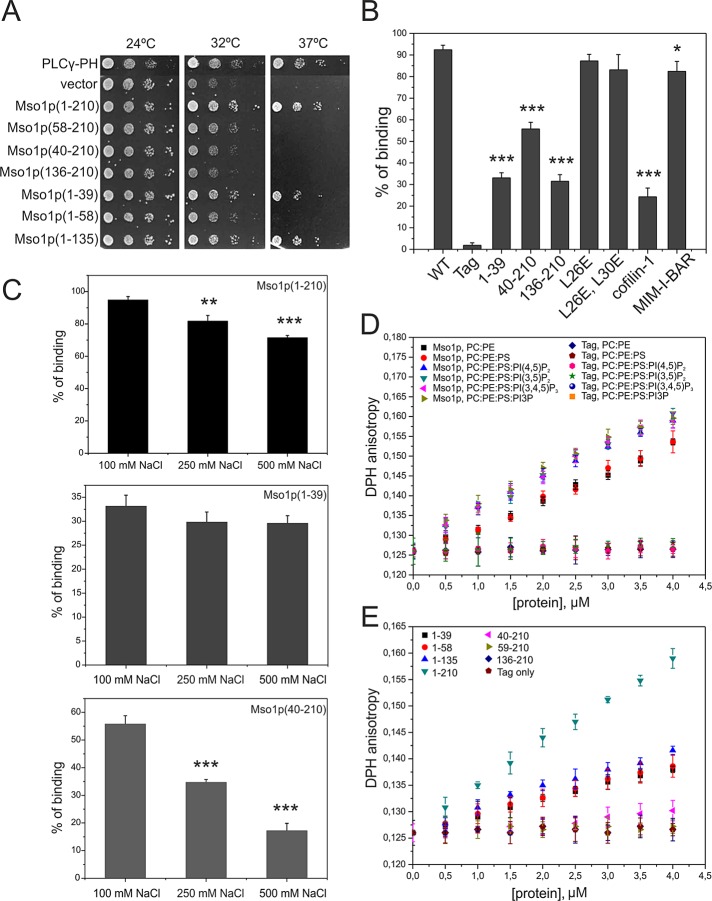
FIGURE 1:
Mso1p interacts with membranes through two distinct binding sites. (A) Mapping of the Mso1p membrane interaction domain in vivo. The growth of cdc25ts mutant cells transformed with plasmids expressing different Mso1p fragments fused to RasQ61L. RasQ61L fused to PLCγ-PH was used as a positive control. (B) Vesicle cosedimentation assay of Mso1p with PI(4,5)P2-rich membranes. Mso1p binds to the PI(4,5)P2-rich membrane with a high affinity, similar to the well-known membrane-binding protein MIM I-BAR. The Mso1p fragments lacking either the N-terminus or the C-terminus displayed weaker cosedimentation with vesicles and bound membranes with similar affinity to a known actin/lipid-binding protein, cofilin-1. The point mutations disrupting the amphiphatic properties of the N-terminal helix of Mso1p did not significantly affect the membrane-binding affinity. The lipid composition was POPC:POPE:POPS:PIP2 = 50:20:20:10. The final concentrations of proteins and lipids were 3 and 500 μM, respectively. Error bars represent SD. Student’s t test, *p < 0.05 and ***p < 0.001. (C) The salt dependence of Mso1p cosedimented with PIP2-containing vesicles. The membrane binding of Mso1p C-terminus was salt dependent (residues 40–210). In contrast, the membrane binding of the full-length Mso1p was only mildly affected by salt, and the N-terminal fragment (residues 1–39) showed negligible salt dependence. Student’s t test, **p < 0.01 and ***p < 0.001. (D) The effect of Mso1p on lipid bilayer fluidity as measured by DPH anisotropy. Mso1p increased the DPH anisotropy in both zwitterionic and negatively charged membranes, whereas the tag alone did not display detectable effects on DPH anisotropy. Phosphoinositides enhanced the effects of Mso1p on the changes of membrane fluidity. The lipid composition is indicated in the figure, and the total lipid concentration was 40 μM. For PC:PE and PC:PE:PS membranes the compositions were 80:20 and 60:20:20, respectively. (E) The effect of Mso1p fragments on membrane fluidity in the DPH anisotropy assay. The Mso1p fragments lacking the C-terminus of the protein displayed reduced effects on DPH anisotropy. However, the mutants lacking the N-terminal region of the protein completely lost the ability to increase DPH anisotropy, suggesting that the N-terminal region is important for membrane insertion and the C-terminal region of Mso1p facilitates the membrane insertion. The lipid composition used was POPC:POPE:POPS:PI(4,5)P2 = 50:20:20:10.