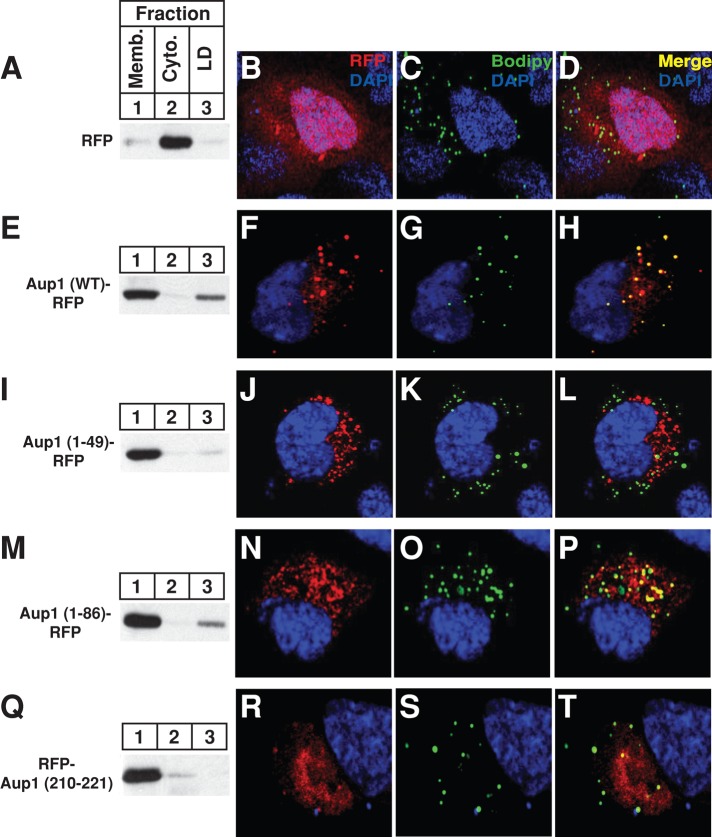
FIGURE 5:
Localization of Aup1 to lipid droplets. CHO-7 cells were set up for subcellular fractionation (A, E, I, M, and Q) on day 0 at 6 × 105 cells/60-mm dish in medium B containing 5% LPDS. On day 1, the cells were transfected with 2 μg/dish pCMV-RFP, -Aup1-RFP, -Aup1(1–49)-RFP, -Aup1(1–86)-RFP, or -Aup1(210–221)-RFP, as indicated and described in the legend to Figure 1, and incubated for 16 h at 37°C in medium A containing 5% dFCS and 100 μM oleate–bovine serum albumin. The cells were then incubated for 2 h at 37°C in sterol-depleting medium containing 10 μM MG-132, after which they were harvested and subjected to subcellular fractionation. Aliquots of the resulting membrane, cytosol, and lipid droplet fractions were analyzed by immunoblot with anti-RFP IgG. For confocal microscopy, SV-589 cells were set up on day 0 at 1.5 × 105 cells/well of six-well plates with glass coverslips in medium B containing 10% FCS. On day 1, cells were transfected with 2 μg/dish pCMV-RFP, -Aup1-RFP, -Aup1(1-49)-RFP, -Aup1(1-86)-RFP, or -Aup1(210-221)-RFP, as indicated, and subsequently incubated at 37°C in medium B containing 10% dFCS. The cells were then subjected to MG-132 treatment in medium B containing 10% dFCS as described above. Cells were subsequently fixed, permeabilized, and stained with 5 μg/ml DAPI to visualize nuclei (blue) and 10 μg/ml Bodipy 493/503 to visualize lipid droplets (green). Images were taken using a Zeiss LSM510 laser-scanning confocal microscope with a META spectral detector, a Chameleon XR NIR laser (Bitplan, St. Paul, MN), and a Zeiss 63×, oil-immersion objective.