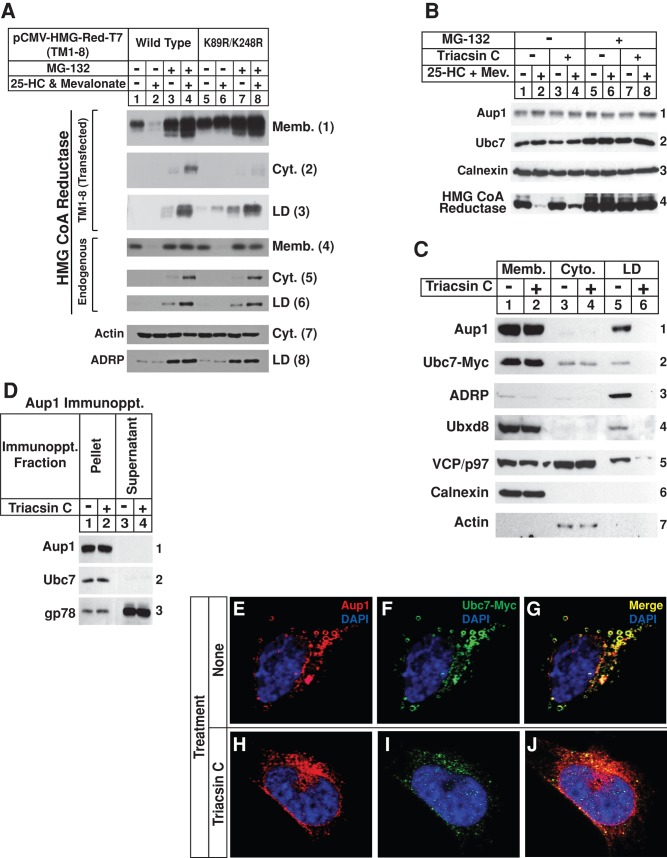
FIGURE 7:
Sterol-induced ubiquitination of HMG CoA reductase occurs in lipid droplet–associated ER membranes. (A) CHO-K1 cells were set up on day 0 at 5 × 105 cells/100-mm dish in medium A containing 5% FCS, transfected on day 2 with 5.82 μg/dish of wild-type or K89R/K248R versions of pCMV-HMG-Red-T7(TM1-8) together with 0.18 μg/dish of pCMV-Insig-1-Myc, as indicated, and depleted of sterols as described in the legend to Figure 1. The cells were then subjected to treatment with sterol-depleting medium containing 10 μM MG-132 in the absence or presence of 1 μg/ml 25-HC plus 10 mM mevalonate as indicated. After 5 h at 37°C, the cells were harvested and subjected to subcellular fractionation. Aliquots of membrane (Memb.), cytosol (Cyt.), and lipid droplet (LD) fractions were subjected to SDS–PAGE, which was followed by immunoblot analysis with anti-T7 IgG (against transfected reductase), IgG-A9 (against endogenous reductase), anti-actin IgG, and anti-ADRP IgG. (B–D) SV-589/pUbc7-Myc cells were set up on day 0 at 8 × 106 cells/100-mm dish in medium B containing 10% FCS. On day 2, the cells were depleted of sterols through incubation for 16 h at 37°C in medium B containing 10% dFCS, 50 μM compactin, and 50 μM mevalonate. Cells were subsequently treated with the identical medium containing 10 μM triacsin C for 3–4 h at 37°C, after which they received 10 μM MG-132 and were further incubated for an additional 3–3.5 h. Cells were then harvested and subjected to either subcellular fractionation (B and C) or anti-Aup1 immunoprecipitation (D). Aliquots of resulting membrane, cytosol, lipid droplet, immunoprecipitation pellet, and supernatant fractions were subjected to SDS–PAGE and immunoblot analysis with IgG-A9 (against reductase), anti-Aup1 IgG, IgG-9E10 (against Ubc7), anti-calnexin IgG, IgG-740F (against gp78), anti-ADRP IgG, anti-Ubxd8 IgG, and anti-actin IgG. (E–J) SV-589/pUbc7-Myc cells were set up for experiments on day 0 and depleted of sterols on day 2 as described above. Following sterol depletion, the cells were re-fed medium B containing 10% LPDS, 50 μM compactin, and 10 μM triacsin C. After 4 h at 37°C, cells received 10 μM MG-132 and were incubated for an additional 2 h. The cells were subsequently fixed, permeabilized, and stained with 5 μg/ml DAPI to visualize nuclei (blue) and 2–5 μg/ml anti-Aup1 IgG together with 15 μg/ml Alexa Fluor 594 donkey anti–rabbit IgG to visualize Aup1 (red) or 15 μg/ml IgG-9E10 together with 15 μg/ml Alexa Fluor 488 donkey anti–mouse IgG to visualize Ubc7-Myc (green), and analyzed by confocal microscopy.