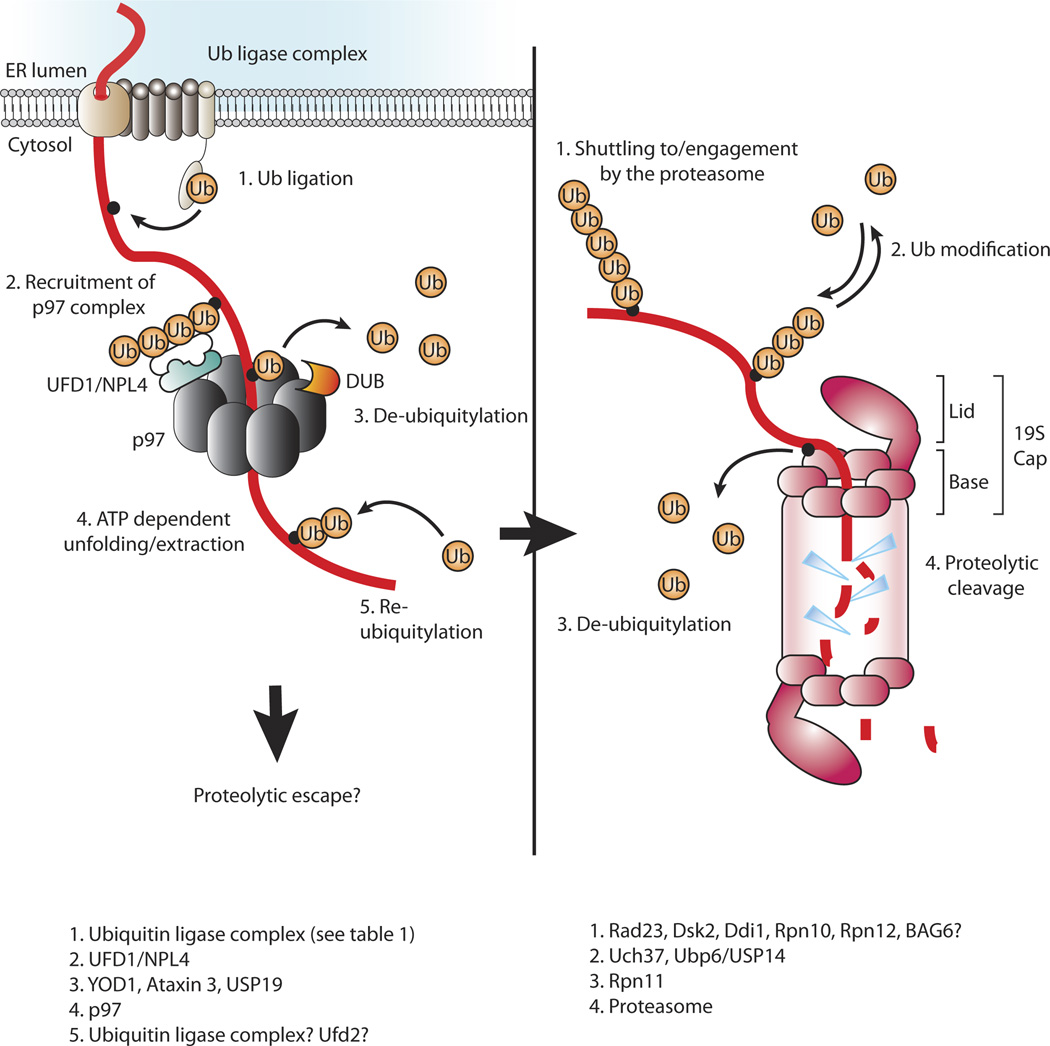
Figure 2. Protein dislocation and/or degradation.
Left panel: A proposed model of a dislocated protein that is ubiquitylated at the ER membrane and consequently engaged by p97 via NFD1/NPL4. A de-ubiquitylating enzyme cleaves ubiquitin to allow threading of the polypeptide through the central pore of p97. A hypothesized re-ubiquitylation step post-p97 then facilitates proteasomal targeting. Right panel: A poly-ubiquitin tag targets the protein for proteasomal degradation. The poly-ubiquitin chain is probably modified before it is finally removed to allow threading of the polypeptide through the central pore of the base of the 19S cap and into the proteolytic chamber of the 20S proteasome core particle.