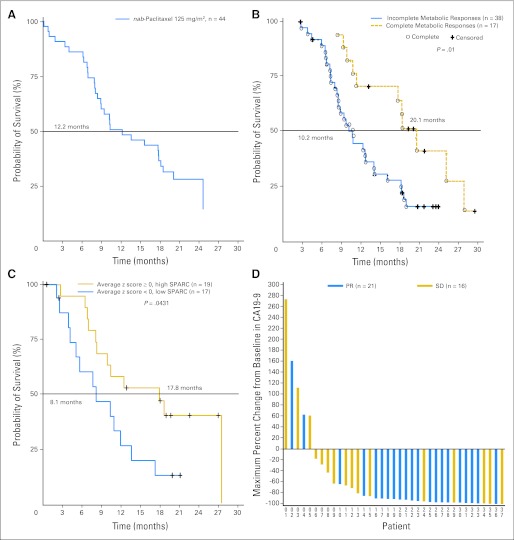
Fig 1.
(A) Median overall survival in patients receiving 125 mg/m2 of albumin-bound (nab) paclitaxel followed by 1,000 mg/m2 of gemcitabine. (B) Median overall survival correlated with a complete metabolic response compared with baseline, defined according to the European Organisation for Research and Treatment of Cancer criteria by the absence of [18F]fluorodeoxyglucose uptake (cohorts 1 and 2). (C) Median survival correlated with secreted protein acidic and rich in cysteine (SPARC; all cohorts). (D) Maximum percentage change in CA19-9 levels in patients receiving 125 mg/m2 of nab-paclitaxel followed by 1,000 mg/m2 of gemcitabine. PR, partial response; SD, stable disease.