Case Report
A 53-year-old white woman with generalized lymphadenopathy was diagnosed with follicular lymphoma, grades 1 to 2, stage III. Three months later, she was started on six cycles of rituximab, cyclophosphamide, vincristine, and prednisone therapy with minimal response. A repeat lymph node biopsy approximately 1 year later revealed persistent follicular lymphoma (grades 1 to 2) after which the patient received six cycles of bendamustine therapy. Epstein-Barr virus (EBV)–encoded RNA in situ hybridization (EBER-ISH) on the lymph node biopsy failed to demonstrate EBV infection.
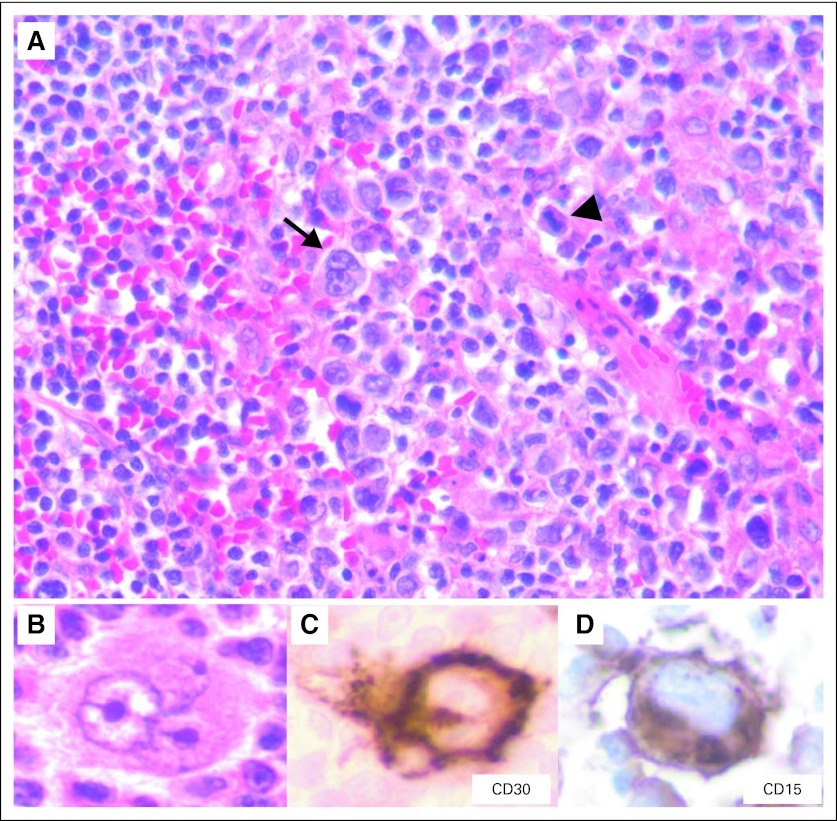
Approximately 1 year after the initiation of bendamustine therapy, the patient returned with progressively enlarging cervical lymph nodes. Complete clinical staging was performed along with a bone marrow and lymph node biopsy. Radiologic work-up revealed enlarged axillary/supraclavicular and pretracheal lymph nodes (the largest measured 3.5 cm). There was no evidence of hepatosplenomegaly or lymphadenopathy elsewhere. A bone marrow biopsy revealed paratrabecular lymphoid aggregates consistent with bone marrow involvement by lymphoma. A right cervical lymph node biopsy demonstrated follicular lymphoma composed of a mixture of centrocytes and centroblasts (> 15/high-power field in most follicles) that was consistent with grade 3A follicular lymphoma. A few follicles revealed large neoplastic cells with classic Reed-Sternberg (RS) and Hodgkin cell (mononuclear variant) -like morphology (Figs 1A and 1B; arrow indicates Hodgkin-like cell). In addition, several mummified cells (typically seen in Hodgkin lymphoma) were also identified (Fig 1A, arrowhead). Although there were a few scattered histiocytes, neutrophils, eosinophils, and plasma cells, the extent of the infiltrate was less than what would be expected for typical Hodgkin lymphoma. No fibrous bands were seen.
Fig 1.
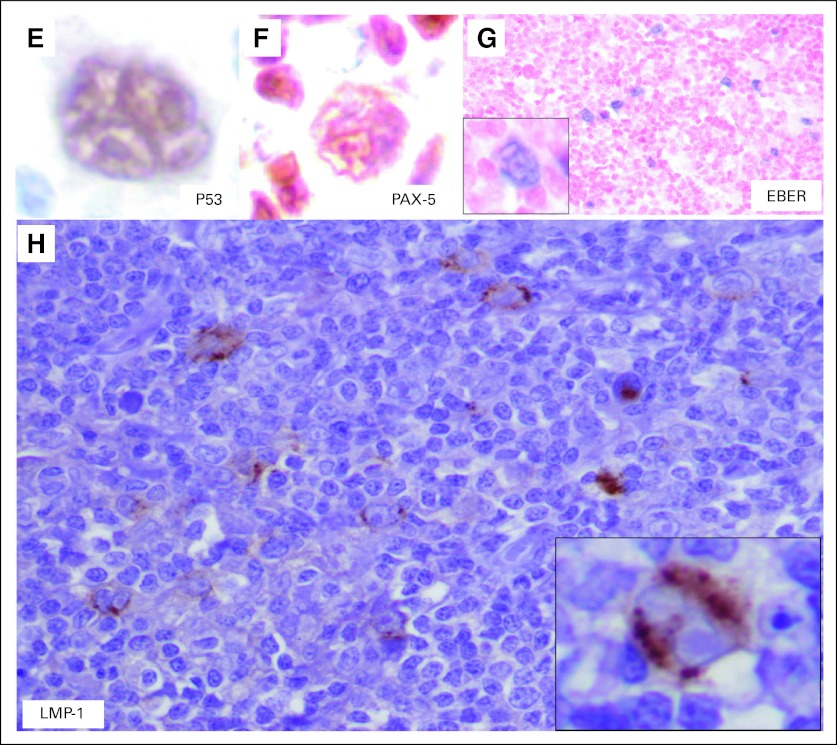
Immunohistochemical studies revealed that the follicular cells were positive for CD20, CD10, BCL6, and BCL2, which was consistent with a diagnosis of follicular lymphoma. The large atypical RS-like cells demonstrated a Hodgkin lymphoma–like immunophenotype with positivity for CD30 (Fig 1C), CD15 (Fig 1D), and paired box 5 (PAX-5; dim; Fig 1F). p53 positivity was also seen in the RS-like cells (Fig 1E). Interestingly, EBER-ISH revealed EBV positivity in Hodgkin-like cells as well as in fewer background cells (< 10%) in the neoplastic follicles (Fig 1G; inset shows a high-power view of the EBER-positive Hodgkin-like cell), and latent membrane protein-1 (LMP-1) immunostain was also positive in the larger Hodgkin-like cells (Fig 1H; inset shows a high-power view of the LMP-1–positive Hodgkin-like cell). In addition, these RS-like cells also exhibited variable to weak positivity for CD20, BCL6, CD45, BOB1, and OCT2 (data not shown). The histologic and immunohistochemical findings were consistent with that of an EBV-positive Hodgkin-like CD30+ transformation of follicular lymphoma. The patient subsequently underwent three cycles of salvage therapy with rituximab, ifosfamide, carboplatin, and etoposide followed by a matched, unrelated-donor bone marrow transplant. However, the post-transplant course of the patient was complicated by graft-versus-host disease, microangiopathic hemolytic anemia, pneumonia, and sepsis. After discussion with her family, the patient received comfort measures only and subsequently died approximately 2 months after her bone marrow transplant.
Discussion
Follicular lymphoma is a relatively indolent B-cell lymphoma composed of neoplastic centrocytes and centroblasts. It is characterized by a translocation between chromosomes 14 and 18 resulting in IGH@/BCL2 fusion and overexpression of BCL2 protein. Follicular lymphoma comprises approximately 20% of all lymphomas with a higher incidence in the United States and Europe.1 It is characterized by widespread lymphadenopathy, splenomegaly, and frequent bone marrow involvement (40% to 70%).2,3 Despite the fact that 70% of patients are in stage III or above at diagnosis, the median survival is 8 to 10 years, with many patients not requiring treatment.3 The histologic transformation of low-grade lymphomas, including follicular lymphoma, is a well-described phenomenon with an average risk of transformation of 20% at 5 years and 30% at 10 years.4 The natural course of follicular lymphomas is to transform to a higher-grade lymphoma, most commonly diffuse large B-cell lymphoma and infrequently to Burkitt lymphoma.4 The occurrence of Hodgkin lymphoma subsequent to follicular lymphoma as well as composite lymphomas that consist of Hodgkin lymphoma (with classic phenotype) and follicular lymphoma have been previously described past.5,6
CD30 is a transmembrane glycoprotein and shows sequence homology to members of the tumor necrosis factor–receptor superfamily.7 CD30 expression has been associated with activated T and B cells as well as human T-cell lymphotropic virus-I or II–transformed T cells or EBV-transformed B cells.8,9 CD30 expression has been best characterized on RS cells in Hodgkin lymphoma but has also been described in the context of various other high-grade B- and T-cell lymphomas, notably, for example, anaplastic T-cell lymphoma, subsets of diffuse large B-cell lymphoma, and cutaneous lymphoproliferative disorders.7,10,11
The phenomenon of transformation of follicular lymphoma to CD30+ large-cell lymphoma has been described previously, and a few reports have described the presence of RS-like cells. Bayerl et al12 described two cases of follicular lymphomas with CD30+ RS-like cells that were negative for CD15. In the same study, a clonal relationship was established between centrocytes, centroblasts, and Hodgkin-like cells via polymerase chain reaction (PCR) –based amplification and sequencing of immunoglobulin heavy chain rearrangements.12 Shin et al13 described five cases of follicular lymphoma with RS-like cells, albeit without CD30 and CD15 expression. In another case series published by Alsabeh et al,14 six cases of follicular lymphoma with CD30+ cells were described, although these cells demonstrated anaplastic cell morphology with absent CD15 staining. Other studies have also studied CD30 expression in follicular lymphoma. Piris et al15 described CD30+ cells in follicular lymphoma predominantly around the neoplastic follicles or T-cell areas in a distribution similar to that seen in reactive tonsils and lymph nodes.Gardner et al16 described cases of follicular lymphoma with various number of pleomorphic large cells that stained with CD30. In these reports, Hodgkin-like morphology was not described, and EBV did not appear to play a role in the transformation process.
Other investigators have tried to address this issue by using single-cell PCR to either look for identical IGH@ rearrangement patterns or via the demonstration of IGH@/BCL2 in both follicular lymphoma and Hodgkin lymphoma components. Brauninger et al17 and Marafioti et al18 demonstrated a clonal relationship between follicular lymphoma and Hodgkin lymphoma in their cases by using single-cell PCR amplification of the immunoglobulin gene. Similarly, Nakamura et al19 described a case of a follicular lymphoma that progressed to Hodgkin lymphoma (positive for CD20, CD30, and CD15) with a demonstration of chimeric IGH@/BCL2 by PCR in both tumors. However, EBV association was not been reported in any of these reports.
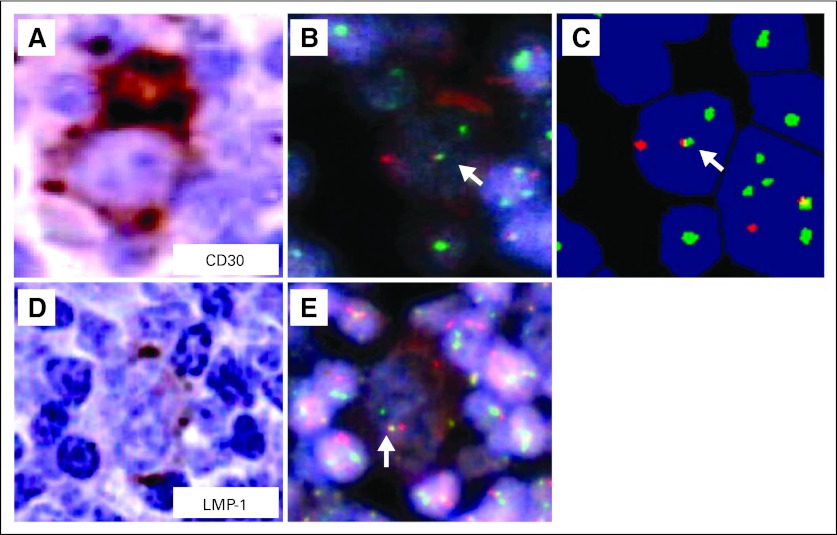
To prove the clonal relationship between the follicular lymphoma and Hodgkin lymphoma–like cells, we adopted a combined IGH@/BCL2 fluorescent in situ hybridization imaging approach with either CD30 or LMP-1 immunohistochemistry by using a BioView Duet image-analysis system (BioView, Billerica, MA). This approach revealed a yellow fusion signal (IGH@/BCL2) with BCL2 (green) and IGH@ (red) probes in the CD30+ cells (Figs 2A to 2C; arrows indicate fusion signals) as well as the presence of fusion signals in LMP-1–positive cells (Figs 2D and 2E; arrow indicates fusion signal). Regarding the background cells, the BioView instrument counted 103 cells (10.55%) with an IGH@/BCL2 fusion and 873 cells (89.4%) without fusion in a total of 976 cells. LMP-1 positivity is seen in EBV type 2 and 3 latency, and EBV-associated Hodgkin lymphomas usually exhibit type 2 latency.
Fig 2.
EBV has been implicated as an etiologic agent in the Hodgkin lymphoma variant of Richter's transformation of chronic lymphocytic leukemia, which usually carries a poor prognosis.20–22 However, to the best of our knowledge, this is the first reported case of an EBV-associated transformation of follicular lymphoma to a CD30+ and CD15+ Hodgkin-like lymphoma. We use the term Hodgkin like as opposed to a bonafide Hodgkin lymphoma transformation because of the less-than-typical inflammatory background as well as variable immunoreactivity for CD45, CD20, OCT2, and BOB1, which are usually negative in classic Hodgkin lymphoma. However, up to 10% of classic Hodgkin lymphoma cases can demonstrate OCT2 and BOB1 reactivity,23 whereas 30% to 40% of cases show CD20 positivity, albeit with weak and variable staining.24 In addition, the RS-like cells were also positive for p53, which has been shown to be associated with progression in follicular lymphoma and poorer overall survival of patients.25 Also, Wang et al26 demonstrated that approximately 22% of Hodgkin lymphomas express p53 with no predilection for a specific subtype. According to the study by Gardner et al16 there is no clear-cut correlation between the percentage of large cells (grade) and CD30 positivity. It would be interesting to determine whether the presence of CD30+ Hodgkin-like cells in the setting of a low-grade follicular lymphoma (grades 1 to 2) might forebode a more-aggressive clinical course and provide prognostic information independent of grade.
In conclusion, we describe a case of transformation of a low-grade follicular lymphoma to a grade 3A follicular lymphoma in which there was an associated CD30+ large-cell lymphoma that exhibited Reed-Sternberg–like morphology and immunophenotype. We were able to demonstrate a clonal relationship between the follicular lymphoma and the Hodgkin like lymphoma. Regarding the role of EBV, there were several large Hodgkin-like cells as well as smaller cells with positive EBER-ISH staining. LMP-1 was positive to a greater extent in the Hodgkin-like cells, which also carried the IGH@/BCL2 translocation.
ACKNOWLEDGMENT
We thank Stefania Pittaluga, MD, PhD from the Laboratory of Pathology, National Cancer Institute, Bethesda, MD, for expert consultation.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
REFERENCES
- 1.Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non-Hodgkin's lymphomas: Distributions of the major subtypes differ by geographic locations—Non-Hodgkin's Lymphoma Classification Project. Ann Oncol. 1998;9:717–720. doi: 10.1023/a:1008265532487. [DOI] [PubMed] [Google Scholar]
- 2.The Non-Hodgkin's Lymphoma Classification Project. A clinical evaluation of the international lymphoma study group classification of non-Hodgkin's lymphoma. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- 3.Salles GA. Clinical features, prognosis and treatment of follicular lymphoma. Hematology Am Soc Hematol Educ Program. 2007;2007:216–225. doi: 10.1182/asheducation-2007.1.216. [DOI] [PubMed] [Google Scholar]
- 4.Montoto S, Fitzgibbon J. Transformation of indolent B-cell lymphomas. J Clin Oncol. 2011;29:1827–1834. doi: 10.1200/JCO.2010.32.7577. [DOI] [PubMed] [Google Scholar]
- 5.Travis LB, Gonzalez CL, Hankey BF, et al. Hodgkin's disease following non-Hodgkin's lymphoma. Cancer. 1992;69:2337–2342. doi: 10.1002/1097-0142(19920501)69:9<2337::aid-cncr2820690923>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez CL, Medeiros LJ, Jaffe ES. Composite lymphoma: A clinicopathologic analysis of nine patients with Hodgkin's disease and B-cell non-Hodgkin's lymphoma. Am J Clin Pathol. 1991;96:81–89. doi: 10.1093/ajcp/96.1.81. [DOI] [PubMed] [Google Scholar]
- 7.Smith CA, Gruss HJ, Davis T, et al. CD30 antigen, a marker for Hodgkin's lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell. 1993;73:1349–1360. doi: 10.1016/0092-8674(93)90361-s. [DOI] [PubMed] [Google Scholar]
- 8.Stein H, Mason DY, Gerdes J, et al. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: Evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66:848–858. [PubMed] [Google Scholar]
- 9.Andreesen R, Osterholz J, Löhr GW, et al. A Hodgkin cell-specific antigen is expressed on a subset of auto- and alloactivated T (helper) lymphoblasts. Blood. 1984;63:1299–1302. [PubMed] [Google Scholar]
- 10.Gruss HJ, Pinto A, Gloghini A, et al. CD30 ligand expression in nonmalignant and Hodgkin's disease-involved lymphoid tissues. Am J Pathol. 1996;149:469–481. [PMC free article] [PubMed] [Google Scholar]
- 11.Oflazoglu E, Grewal IS, Gerber H. Targeting CD30/CD30L in oncology and autoimmune and inflammatory diseases. Adv Exp Med Biol. 2009;647:174–185. doi: 10.1007/978-0-387-89520-8_12. [DOI] [PubMed] [Google Scholar]
- 12.Bayerl MG, Bentley G, Bellan C, et al. Lacunar and reed-sternberg-like cells in follicular lymphomas are clonally related to the centrocytic and centroblastic cells as demonstrated by laser capture microdissection. Am J Clin Pathol. 2004;122:858–864. doi: 10.1309/PMR8-6PHK-K4J3-RUH3. [DOI] [PubMed] [Google Scholar]
- 13.Shin SS, Ben-Ezra J, Burke JS, et al. Reed-Sternberg-like cells in low-grade lymphomas are transformed neoplastic cells of B-cell lineage. Am J Clin Pathol. 1993;99:658–662. doi: 10.1093/ajcp/99.6.658. [DOI] [PubMed] [Google Scholar]
- 14.Alsabeh R, Medeiros LJ, Glackin C, et al. Transformation of follicular lymphoma into CD30-large cell lymphoma with anaplastic cytologic features. Am J Surg Pathol. 1997;21:528–536. doi: 10.1097/00000478-199705000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Piris M, Gatter KC, Mason DY. CD30 expression in follicular lymphoma. Histopathology. 1991;18:25–29. doi: 10.1111/j.1365-2559.1991.tb00810.x. [DOI] [PubMed] [Google Scholar]
- 16.Gardner LJ, Polski JM, Evans HL, et al. CD30 expression in follicular lymphoma. Arch Pathol Lab Med. 2001;125:1036–1041. doi: 10.5858/2001-125-1036-CEIFL. [DOI] [PubMed] [Google Scholar]
- 17.Bräuninger A, Hansmann ML, Strickler JG, et al. Identification of common germinal-center B-cell precursors in two patients with both Hodgkin's disease and non-Hodgkin's lymphoma. N Engl J Med. 1999;340:1239–1247. doi: 10.1056/NEJM199904223401604. [DOI] [PubMed] [Google Scholar]
- 18.Marafioti T, Hummel M, Anagnostopoulos I, et al. Classical Hodgkin's disease and follicular lymphoma originating from the same germinal center B cell. J Clin Oncol. 1999;17:3804–3809. doi: 10.1200/JCO.1999.17.12.3804. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura N, Ohshima K, Abe M, et al. Demonstration of chimeric DNA of bcl-2 and immunoglobulin heavy chain in follicular lymphoma and subsequent Hodgkin lymphoma from the same patient. J Clin Exp Hematop. 2007;47:9–13. doi: 10.3960/jslrt.47.9. [DOI] [PubMed] [Google Scholar]
- 20.Momose H, Jaffe ES, Shin SS, et al. Chronic lymphocytic leukemia/small lymphocytic lymphoma with Reed-Sternberg-like cells and possible transformation to Hodgkin's disease. Mediation by Epstein-Barr virus. Am J Surg Pathol. 1992;16:859–867. doi: 10.1097/00000478-199209000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Petrella T, Yaziji N, Collin F, et al. Implication of the Epstein-Barr virus in the progression of chronic lymphocytic leukaemia/small lymphocytic lymphoma to Hodgkin-like lymphomas. Anticancer Res. 1997;17:3907–3913. [PubMed] [Google Scholar]
- 22.Rubin D, Hudnall SD, Aisenberg A, et al. Richter's transformation of chronic lymphocytic leukemia with Hodgkin's-like cells is associated with Epstein-Barr virus infection. Mod Pathol. 1994;7:91–98. [PubMed] [Google Scholar]
- 23.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (ed 4) Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 24.Schmid C, Pan L, Diss T, et al. Expression of B-cell antigens by Hodgkin's and Reed-Sternberg cells. Am J Pathol. 1991;139:701–707. [PMC free article] [PubMed] [Google Scholar]
- 25.Pennanen H, Kuittinen O, Soini Y, et al. Prognostic significance of p53 and matrix metalloproteinase-9 expression in follicular lymphoma. Eur J Haematol. 2008;81:289–297. doi: 10.1111/j.1600-0609.2008.01113.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Taylor CR. Apoptosis and cell cycle-related genes and proteins in classical Hodgkin lymphoma: Application of tissue microarray technique. Appl Immunohistochem Mol Morphol. 2003;11:206–213. doi: 10.1097/00129039-200309000-00002. [DOI] [PubMed] [Google Scholar]