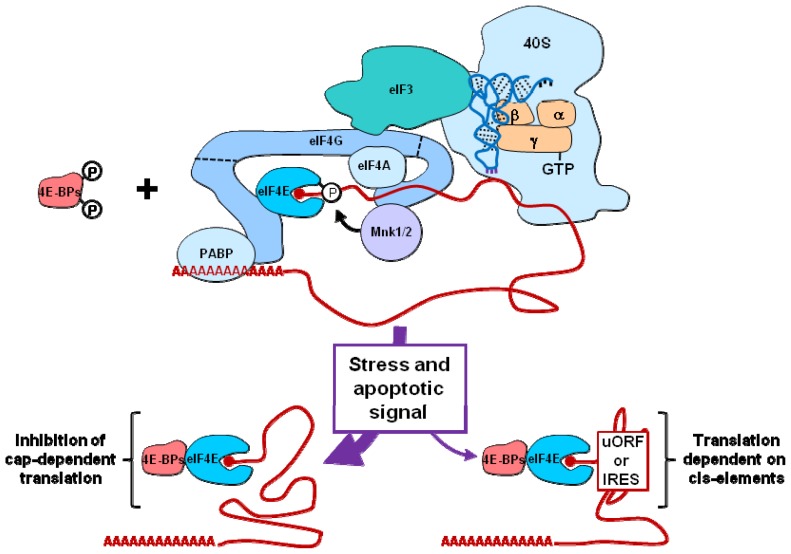
Figure 2.
Translational functions of the eIF3 and eIF4F complexes and inhibition by the 4E-BPs. The eIF4F complex is composed of eIF4E, eIF4G and eIF4A. The interaction between eIF4F (bound to the cap structure via its eIF4E subunit) and eIF3 (bound to the 40S ribosomal particle) complexes permits ribosome docking at the 5′ extremity of capped mRNAs. The melting of mRNA structures by the RNA helicase eIF4A facilitates ribosome binding to mRNA, and the interaction between eIF4G and PABP results in mRNA circularization, a feature that is believed to enhance mRNA translation. eIF4G recruits the eIF4E kinases MNK1 and MNK2. In stressed cells, the dephosphorylation of 4E-BPs disrupts the eIF4E-eIF4G interaction, inhibiting general cap-dependent translation (left arrow). However the translation of selected mRNAs is still possible due to the existence of either uORFs or IRESes (right arrow). See text for details.