Figure 1.
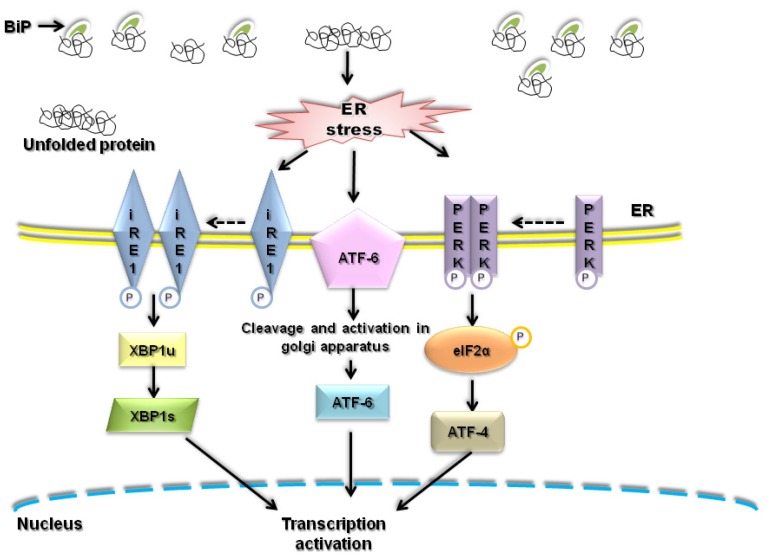
Endoplasmatic reticulum (ER) stress and unfolded protein response (UPR) induction. During the pathological conditions, proteins are aggregated causing accumulation of misfolded proteins in the ER lumen. This accumulation of misfolded proteins enhances UPR. During the initiation of UPR, BiP preferentially binds to the unfolded proteins, driving its equilibrium binding away from inositol requiring enzyme 1 (IRE-1), PKR-like endoplasmic reticulum kinase (PERK) and activating transcription factor 6 (ATF-6) proteins which are considered as initiators of the three main signaling cascades of UPR. IRE-1 protein dimerizes and activates its protein kinase activity (for autophosphorylation) and its endoribonuclease activity. It cleaves X-box-binding protein 1 (XBP1) mRNA to remove a small intron, converting unspliced (XBP1u) to spliced form (XBP1s), resulting in yielding a more potent transcriptional activator. Similarly, PERK also dimerizes and activates to phosphorylate eukaryotic initiation factor 2 (eIF2) on the α-subunit. This action selectively translates ATF-4 mRNA which further induces more transcriptional activator. Activation of ATF-6 allows it to translocate to the golgi apparatus, where it is cleaved by a protease, changing it to the active cytosolic ATF-6 fragment. This fragment migrates to the nucleus, activating the transcription of UPR target genes.