Abstract
Members of some social insects adjust their behaviours depending upon social context. Such plasticity allows colonies to sustain efficiency of the whole without the cost of additional production of individuals or delayed responses to perturbations. Using the recently discovered social clonal stage of trematode parasites, we investigated whether members of the reproductive caste adjust their defensive behaviour according to the local availability of non-reproductive defensive specialists, and if so whether the plasticity affects the short-term reproductive success of reproductive morphs. In vitro experiments demonstrated plasticity in competitive interactions of the reproductive morphs depending on the number of non-reproductive defensive specialists present nearby, which lead to differences in reproductive output at the individual level. This study provides support for the benefit of maintaining non-reproductive morphs in competitive situations, arising through socially mediated behavioural plasticity.
Keywords: behavioural plasticity, social trematodes, Philophthalmus sp.
1. Introduction
The shared interests of genetically identical entities promote division of labour, either through cell specialization in multi-cellular organisms or the formation of distinct physical castes in social organisms. In the latter, adaptive adjustments to varying environmental conditions are more feasible than in the former, via altered investments in individuals to maintain colony efficiency. This is achieved through alteration of caste ratios, where caste allocation decisions vary according to environmental fluctuations [1,2] or through morphological and behavioural plasticity [3].
Behavioural plasticity allows colonies to sustain ergonomic efficiency of the whole without paying the cost of producing additional individuals [4]. Such plasticity can be manifested in relation to the density of other colony members; indeed, some social insects show plasticity in morphologies and behaviours in response to changing caste ratios [3]. For example, termite workers demonstrate increased aggression towards intruders when soldiers are removed from the colony [4,5].
Adaptive shifts in behaviours according to social cues should occur when accompanied by positive fitness consequences, and made possible by proximate mechanisms, such as kin recognition and variable behavioural traits. Although division of labour through formation of physical castes occurs in diverse organisms (insects [3], snapping shrimps [6], sea anemones [7], mole rats [8], parasitic trematodes [9,10]), behavioural plasticity of individual caste members depending upon social context has only been documented in insects [4,5]. In the recently discovered social trematodes, we investigate whether the reproductive morphs of Philophthalmus sp. show plastic behaviours determined by the proportion of non-reproductive defensive specialists in the colony.
In the clonal intermediate stage of social trematodes, one morph produces cercariae (motile larvae that leave the gastropod host), whereas the second morph is considerably smaller and reproductively inactive [9,10]. Several lines of empirical evidence [9] suggest that non-reproductive morphs are defensive specialists. In addition, direct consumption of co-infecting species by non-reproductives has been reported in Philophthalmus sp. [10]. Furthermore, the defensive specialist hypothesis is consistent with interspecific competition for resources and space exerting strong selection on trematodes infecting gastropod intermediate hosts [11,12], as shown in our study system [13].
Despite the presence of defensive specialists, consumption of intra-host competitors by reproductive morphs has been reported in social Himasthla sp. [9] and in other social trematodes without distinct castes [14,15]. Although the reproductive morphs of Philophthalmus sp. have not been documented to interact with their intra-host competitors [10], we observed that reproductive morphs of Philophthalmus sp. feed on the trematode competitor Maritrema novaezealandensis. Assuming that (i) reproductive morphs are less suited for, yet capable of attacking competitors, and (ii) the colony ultimately benefits from alleviation of competition, flexible defensive behaviour by reproductive morphs should be adaptive as a means to resist or exclude competitors. By contrast, defensive behaviour may present a fitness cost to the colony, because the extra energy required can be diverted towards reproduction when specialized defensive morphs are abundant.
We test whether the reproductives of Philophthalmus sp. adjust their behaviour according to the number of non-reproductives, and whether the defensive behaviour of reproductive morphs affects their reproductive output. We thus demonstrate socially mediated behavioural plasticity in colonial organisms other than insects.
2. Material and methods
(a). Study organisms
Philophthalmus sp. is the second most common trematode infecting the mud snail, Zeacumantus subcarinatus, as first intermediate host at our study site with up to 8 per cent prevalence, following M. novaezealandensis which infects over 50 per cent of individuals in the snail population; they co-occur in 12 per cent of the hosts [16]. Both trematode species multiply asexually in the snail, forming masses of clonal stage collectively called parthenitae that produce free-swimming dispersal stages (cercariae), which then leave the snail. Parthenitae of Philophthalmus sp., are called rediae and possess mouthparts, and occur as both non-reproductive and reproductive morphs; those of M. novaezealandensis are of a different type known as sporocysts and lack mouthparts. Snails were collected in February and March 2012 from Otago Harbor, South Island, New Zealand (45°520′ S, 170°420′ E). Live Philophthalmus sp. rediae and M. novaezealandensis sporocysts were obtained by dissection of infected snails, and used in the following experiments (for more detail, see the electronic supplementary material, appendix S1).
(b). Behavioural plasticity versus number of defensive specialists
Parthenitae were cultured in wells of 12-well culture plates, each filled with 1 ml culture medium previously optimized to culture both trematode species for up to 56 days [17]. The medium is briefly described in the electronic supplementary material, appendix S1. Philophthalmus sp. rediae were exposed to three different treatments with a fixed number of reproductives (six rediae) and of M. novaezealandensis sporocysts (i.e. approx. 30 sporocysts), but with differing numbers of the non-reproductive rediae (two, six and 18, respectively) to reflect caste ratios in natural populations (non-reproductive : reproductive = 0.48–4.18, T. Kamiya 2012, personal observation). Rediae from a single snail were used for one replicate of each treatment, and M. novaezealandensis sporocysts were also taken from a separate single snail for each replicate, to homogenize genotypes across treatments. Philophthalmus rediae tend to attach to sporocysts using a posterior protrusion and swing their body to feed on M. novaezealandensis (for photographs, see the electronic supplementary material, appendix S2). Thus, the proportion of rediae in contact with sporocysts of M. novaezealandensis was recorded daily as a proxy for competitive interactions. Cultures were maintained for 14 days at 18°C under ambient light and replenished with new medium every 3–4 days. The experiment was replicated 15 times using 15 independent Philophthalmus colonies.
The daily proportion of the six reproductive rediae in contact with M. novaezealandensis was arcsine square-root transformed and compared among the treatments differing in the number of non-reproductives in a linear mixed model that included day as mixed effects using the lme4 package in R (v. 2.14.1) [18]. The initial volume of rediae, calculated as cylinders from micrographs on ImageJ v. 1.45s and averaged across all six rediae per replicate, was also included as a predictor. In addition, the potential influence of differences among genotypes of rediae was controlled by including clone identity as a random effect. Although we recognize the potential confounding effect of different densities of individuals per treatment, this effect should be minimal as the volume of an individual non-reproductive was, on average, only about 1.35 × 10−6 of the volume of the culture medium in a well.
(c). Reproductive output versus competitive interaction
Reproductive rediae of Philophthalmus (12 rediae each from 10 colonies) were individually housed in 96-well culture plates with a standardized number of M. novaezealandensis (i.e. approx. 10 sporocysts) in the culture medium described earlier.
Contact between parthenitae of Philophthalmus and M. novaezealandensis was recorded every 3 h during the day (i.e. 09.00, 12.00, 15.00 and 18.00) for 7 days, as a proxy for competitive interactions. The reproductive output of each redia was scored as the number of cercariae produced over 7 days; when released, Philophthalmus cercariae quickly form cysts attached to well surfaces, which were counted at the end of the experiment.
The cercarial output of Philophthalmus was regressed against the proportion of times the rediae were observed in contact with M. novaezealandensis, using a generalized linear mixed model with negative binomial family, which controlled for zero-inflation of the response variable. Again, the initial volume of individual rediae was included as a fixed effect, and the potential influence of differences among genotypes of rediae was controlled by including clone identity as a random effect.
3. Results
The proportion of time the reproductives were in contact with the competitor decreased as a function of the number of non-reproductives present in the in vitro culture (figure 1 and table 1). In addition, the frequency of competitive interactions decreased as the average volume of reproductives increased (table 1).
Figure 1.
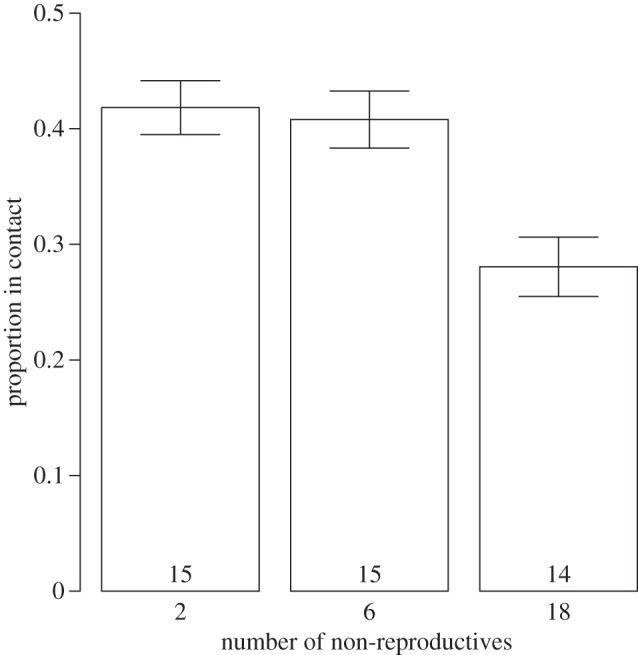
Proportion of reproductive morphs of Philophthalmus sp. seen in contact with the competing Maritrema novaezealandensis over 14 days as a function of differing numbers of non-reproductives in the culture. Sample sizes are shown. The variation in sample sizes occurred owing to contamination of one culture.
Table 1.
Results of a linear mixed model evaluating the relationship between the proportion of reproductive rediae in contact with the competitor, Maritrema novaezealandensis and the number of non-reproductives present. (Genotypes of the rediae, included as a random effect, explained 20% of the variance unaccounted for by the main effects. Bold type indicates significant results (p-value < 0.05).)
| estimate | s.e. | t-value | p-value | |
|---|---|---|---|---|
| intercept | 1.3409 | 0.1491 | 8.994 | <0.001 |
| number of non-reproductives | −0.0093 | 0.0019 | −4.854 | <0.001 |
| volume of reproductives | −11.713 | 1.6520 | −7.090 | <0.001 |
The reproductive output of individual reproductive morphs decreased as a function of the proportion of time they spent in contact with competitors (figure 2), indicating an immediate cost of engaging in competitive interactions (estimate = −0.999, s.e. = 0.322, z-value = −3.10, p = 0.0019).
Figure 2.
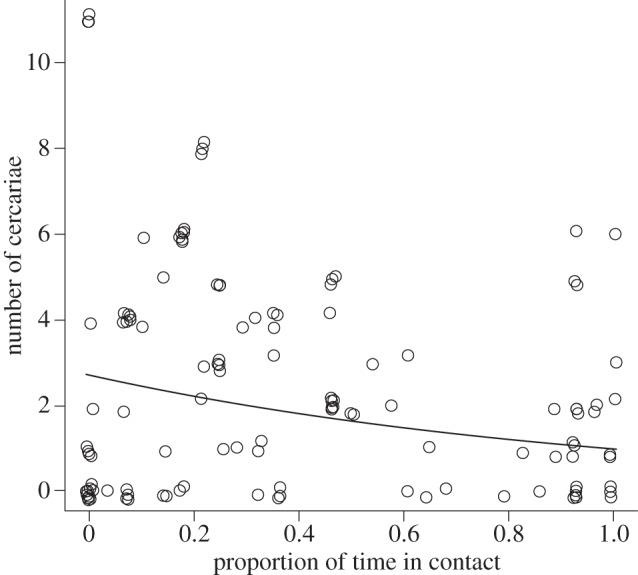
Number of encysted cercariae over 7 days from individual Philophthalmus sp. reproductive rediae, regressed against the proportion of time spent in contact with the competing Maritrema novaezealandensis. Twelve reproductives each from 10 colonies were involved (r2 = 6.7%).
4. Discussion
Our results demonstrate plasticity in the competitive behaviour of the reproductive caste of Philophthalmus sp. depending upon social context. Although possibly incapable of adjustments in response to the other morph's immediate behaviour (see the electronic supplementary material, appendix S3), reproductives engage in fewer competitive interactions with increasing numbers of non-reproductive defensive specialists in the colony. This plastic response was accompanied by an immediate fitness benefit for the individual reproductive morphs. Taken together, relatively high numbers of non-reproductive morphs discourage the reproductive morph from engaging in competitive interactions, which in turn results in a higher reproductive output for the whole colony. Our findings provide a plausible mechanism to explain the benefit of maintaining non-reproductive morphs in competitive situations as reported in our recent studies [13,19].
Engaging in competitive interactions clearly lowers the reproductive output of reproductive rediae, highlighting the cost of this activity. Therefore, when possible for the reproductives to avoid such costly activities (as in the presence of many non-reproductives), the colony as a whole benefits from increased reproduction achieved through more prolific reproductive morphs.
Some ecological traits of Philophthalmus sp. should promote the evolution of socially mediated behavioural plasticity. First, the ratio of non-reproductive to reproductive morphs varies widely [10]. Second, colonies cannot adjust caste ratios according to the presence of the intra-host competitor [10]. Additionally, non-reproductive morphs are distributed unevenly inside the host, with a higher proportion of non-reproductives towards the opening of a snail, which Hechinger et al. [9] describe as ‘the invasion front’, and a lower proportion towards the tip of the snail. This suggests that reproductive morphs are likely to come in direct contact with competitors along with varying numbers of non-reproductive defensive specialists. Therefore, selection should favour defensive behavioural plasticity in reproductives depending upon the availability of nearby non-reproductives. Hechinger et al. [9] also provided indirect evidence for kin recognition mechanisms that are essential for plasticity based on social cues.
Socially mediated behavioural plasticity in trematodes is analogous to the adjustable aggression levels of termite workers towards intruders depending on the presence of nest-mate soldiers [4]. Although the two taxa are distantly related, they share a suite of ecological features that may have led to a convergence of their social organization and behaviour.
Acknowledgements
We thank Manami Iwakura for field assistance; Melanie Lloyd for laboratory assistance; Martin Krkosek and Shinichi Nakagawa for statistical advice; and Katie O'Dwyer for comments on earlier drafts.
References
- 1.Passera L, Roncin E, Kaufmann B, Keller L. 1996. Increased soldier production in ant colonies exposed to intraspecific competition. Nature 379, 630–631 10.1038/379630a0 (doi:10.1038/379630a0) [DOI] [Google Scholar]
- 2.Lecoutey E, Chaline N, Jaisson P. 2011. Clonal ant societies exhibit fertility-dependent shifts in caste ratios. Behav. Ecol. 22, 108–113 10.1093/beheco/arq182 (doi:10.1093/beheco/arq182) [DOI] [Google Scholar]
- 3.Wilson EO. 1971. The insect societies. Cambridge, MA: The Belknap Press of Harvard University Press [Google Scholar]
- 4.Ishikawa Y, Miura T. 2012. Hidden aggression in termite workers: plastic defensive behaviour dependent upon social context. Anim. Behav. 83, 737–745 10.1016/j.anbehav.2011.12.022 (doi:10.1016/j.anbehav.2011.12.022) [DOI] [Google Scholar]
- 5.Roisin Y, Everaerts C, Pasteels JM, Bonnard O. 1990. Caste-dependent reactions to soldier defensive secretion and chiral alarm recruitment pheromone in Nasutitermes princeps. J. Chem. Ecol. 16, 2865–2875 10.1007/bf00979479 (doi:10.1007/bf00979479) [DOI] [PubMed] [Google Scholar]
- 6.Duffy JE. 1996. Eusociality in a coral-reef shrimp. Nature 381, 512–514 10.1038/381512a0 (doi:10.1038/381512a0) [DOI] [Google Scholar]
- 7.Ayre DJ, Grosberg RK. 1996. Effects of social organization on inter-clonal dominance relationships in the sea anemone Anthopleura elegantissima. Anim. Behav. 51, 1233–1245 10.1006/anbe.1996.0128 (doi:10.1006/anbe.1996.0128) [DOI] [Google Scholar]
- 8.Jarvis JUM. 1981. Eusociality in a mammal: cooperative breeding in naked mole-rat colonies. Science 212, 571–573 10.1126/science.7209555 (doi:10.1126/science.7209555) [DOI] [PubMed] [Google Scholar]
- 9.Hechinger RF, Wood AC, Kuris AM. 2011. Social organization in a flatworm: trematode parasites form soldier and reproductive castes. Proc. R. Soc. B 278, 656–665 10.1098/rspb.2010.1753 (doi:10.1098/rspb.2010.1753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung TLF, Poulin R. 2011. Small worms, big appetites: ratios of different functional morphs in relation to interspecific competition in trematode parasites. Int. J. Parasitol. 41, 1063–1068 10.1016/j.ijpara.2011.05.001 (doi:10.1016/j.ijpara.2011.05.001) [DOI] [PubMed] [Google Scholar]
- 11.Kuris AM, Lafferty KD. 1994. Community structure: larval trematodes in snail hosts. Annu. Rev. Ecol. Syst. 25, 189–217 10.1146/annurev.ecolsys.25.1.189 (doi:10.1146/annurev.ecolsys.25.1.189) [DOI] [Google Scholar]
- 12.Poulin R. 2001. Interactions between species and the structure of helminth communities. Parasitology 122, S3–S11 10.1017/s0031182000016991 (doi:10.1017/s0031182000016991) [DOI] [PubMed] [Google Scholar]
- 13.Lloyd MM, Poulin R. 2012. Fitness benefits of a division of labour in parasitic trematode colonies with and without competition. Int. J. Parasitol. 42, 939–946 10.1016/j.ijpara.2012.07.010 (doi:10.1016/j.ijpara.2012.07.010) [DOI] [PubMed] [Google Scholar]
- 14.Martin WE, Adams JE. 1961. Life cycle of Acanthoparyphium-spinulosum Johnston, 1917 (Echinostomatidae, Trematoda). J. Parasitol. 47, 777–782 10.2307/3275470 (doi:10.2307/3275470) [DOI] [PubMed] [Google Scholar]
- 15.Stunkard HW. 1966. Morphology and life history of digenetic trematode Himasthla littorinae sp. (Echinostomatidae). J. Parasitol. 52, 367–372 [Google Scholar]
- 16.Keeney DB, Boessenkool S, King TM, Leung TLF, Poulin R. 2008. Effects of interspecific competition on asexual proliferation and clonal genetic diversity in larval trematode infections of snails. Parasitology 135, 741–747 10.1017/s0031182008004435 (doi:10.1017/s0031182008004435) [DOI] [PubMed] [Google Scholar]
- 17.Lloyd MM, Poulin R. 2011. In vitro culture of marine trematodes from their snail first intermediate host. Exp. Parasitol. 129, 101–106 10.1016/j.exppara.2011.07.009 (doi:10.1016/j.exppara.2011.07.009) [DOI] [PubMed] [Google Scholar]
- 18.R Development Core Team 2011. R: a language and environment for statistical computing, 2.14.1 edn Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 19.Kamiya T, Poulin R. In press. Caste ratios affect the reproductive output of social trematode colonies. J. Evol. Biol. 10.1111/jeb.12062 (doi:10.1111/jeb.12062) [DOI] [PubMed] [Google Scholar]


