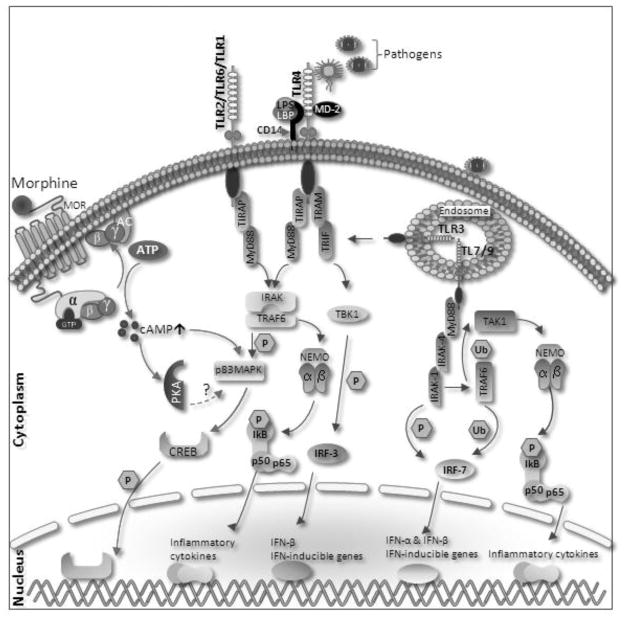
Figure 1.
Proposed mechanism for opioid modulation for TLR. Morphine leads to superactivation of adenylyl cyclase and increase intracellular cAMP levels. cAMP activates p38MAPK in turn leading to CREB (cAMP response element-binding)phosphorylation and nuclear translocation, leading transcription of its target genes. Surface receptor (TLR4, TLR2) and endosomal receptors (TLR7 and TLR9) mediate through MyD88 dependent pathway. Upon activation of TLR 2 and 4 by their cognate ligands, MyD88 recruits IRAK4, thereby allowing the association of IRAK1. IRAK4 then induces the phosphorylation of IRAK1. TRAF6 (Tumour Necrosis Factor-Receptor-Associated Factor-6) is then recruited to the receptor complex, by associating with phosphorylated IRAK1. Phosphorylated IRAK1 and TRAF6 then dissociate from the receptor to form a complex with TBK1 (TGF-Beta-Activated Kinase-1)- a ubiquitin ligases (Ub). This leads to the ubiquitylation of TRAF6, which induces the activation of TAK1. TAK1, in turn, phosphorylates p38MAPK (mitogen activated protein kinase). p38 phosphorylates CREB and induce the expression of its target genes. TAK1 also phosphorylates NEMO (nuclear factor-κB (NF-κB) essential modulator) which having IKK complex (Inhibitor of Kappa Light Polypeptide Gene Enhancer in B-Cells Kinase). The IKK complex then phosphorylates I-KappaB, which leads to its ubiquitylation and subsequent degradation. This allows NF-KappaB to translocate to the nucleus and induce the expression of its target genes. TRIF and TRAM link TLR4 to pathways that lead to TBK1 (TANK Binding Kinase-1) and IRF3 activation (i.e., the MyD88-independent pathway).