Abstract
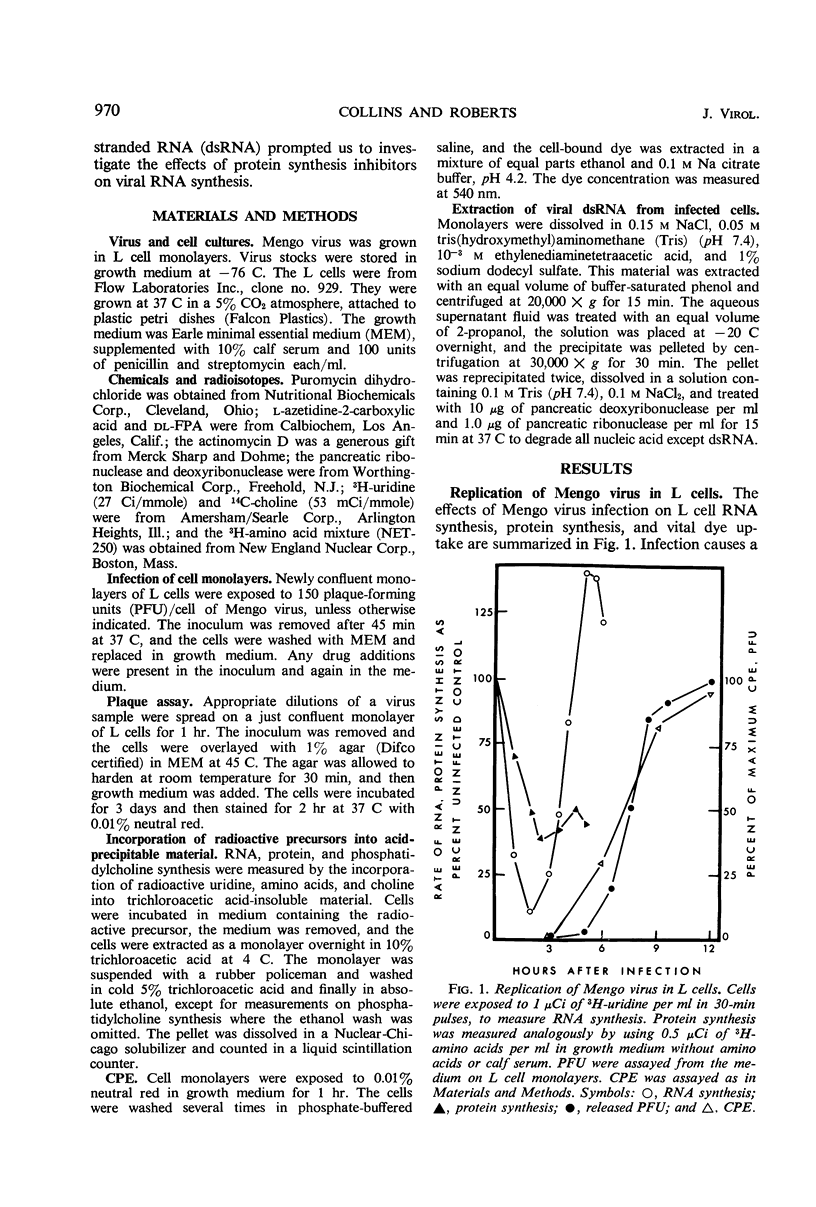
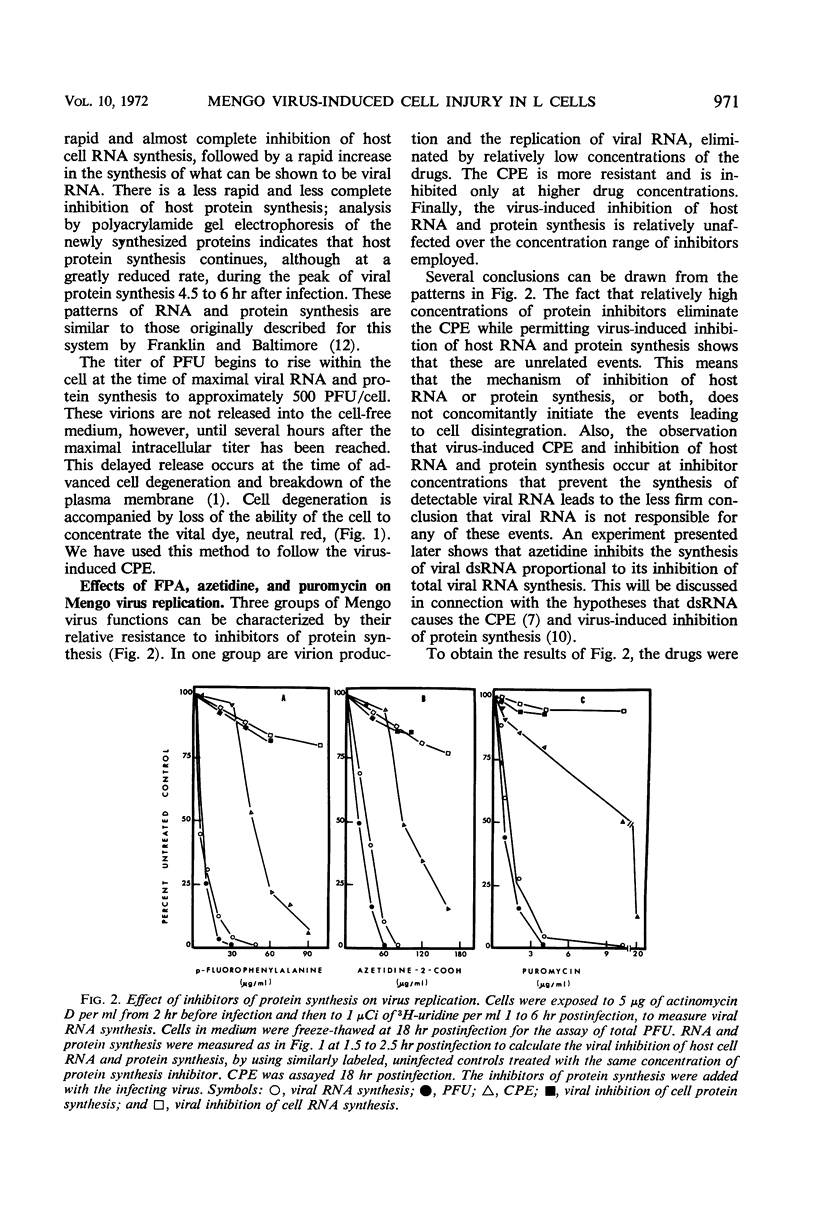
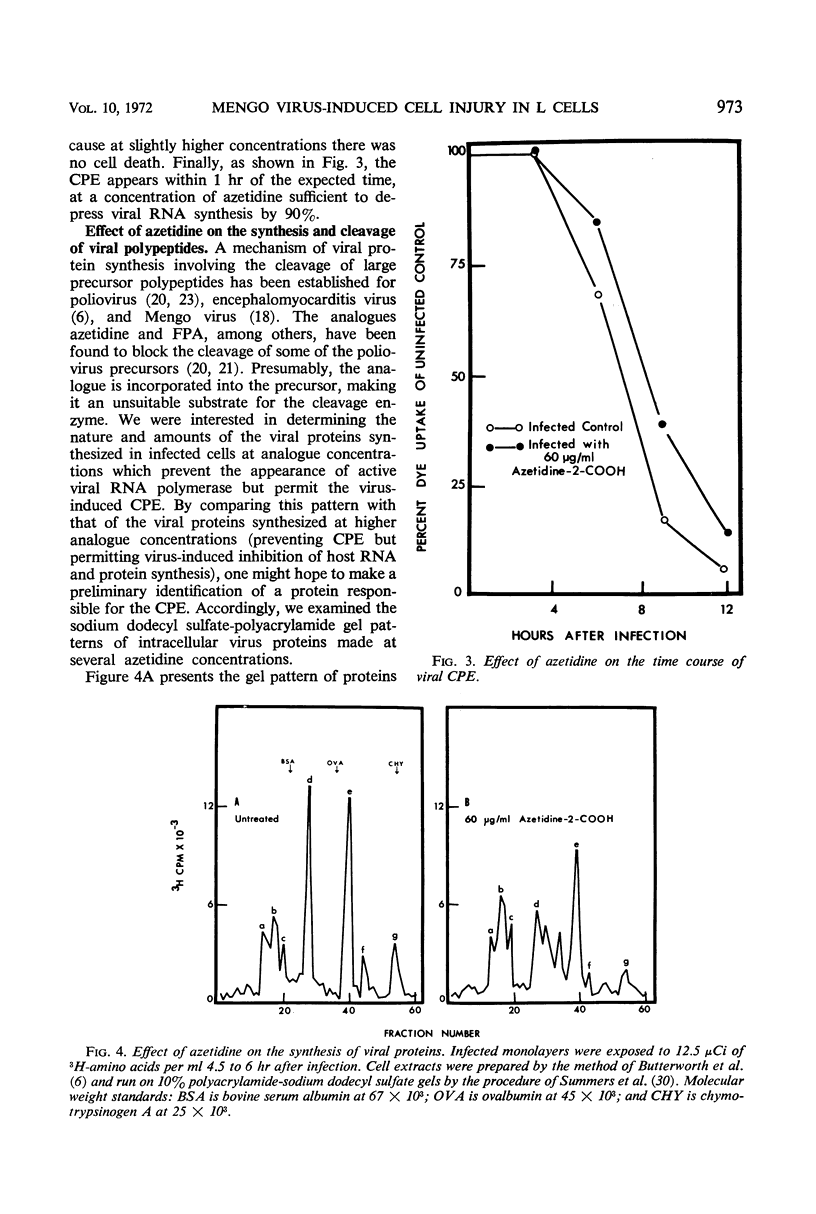
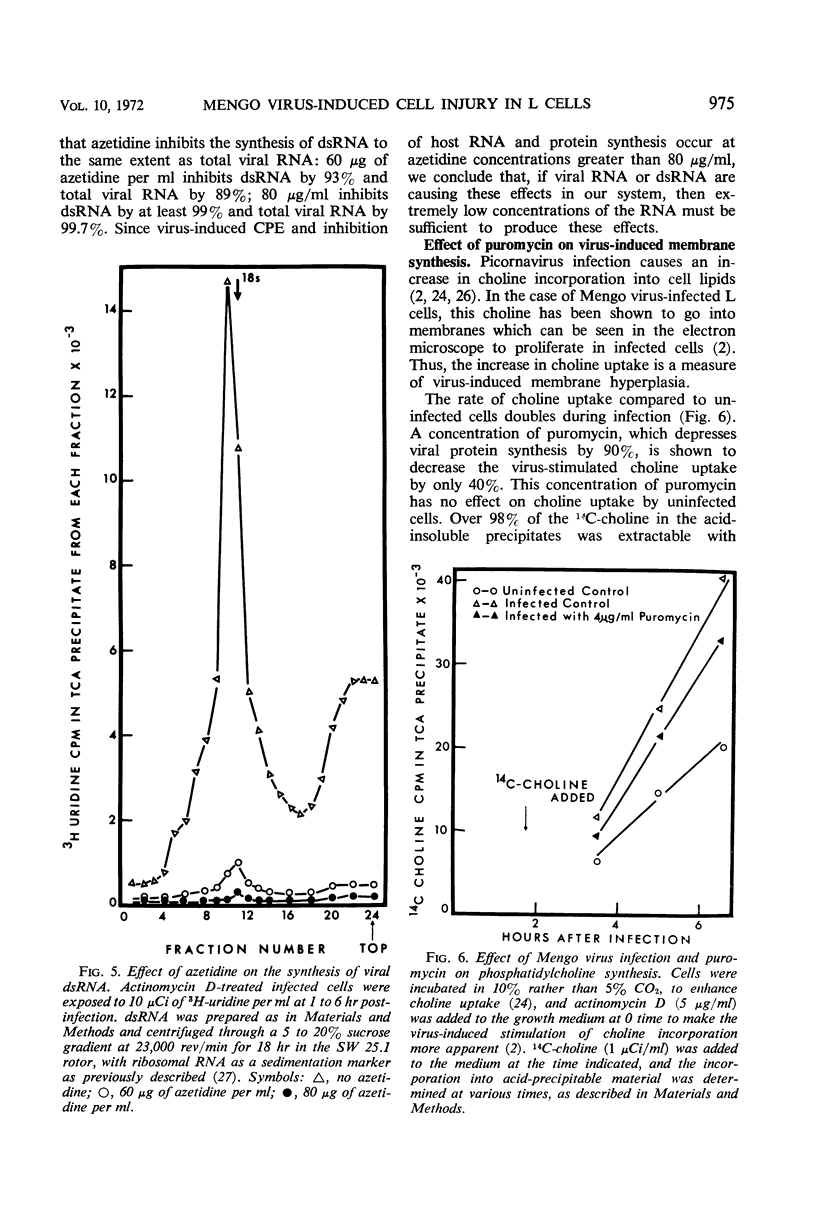
L cells were infected with Mengo virus in the presence of varying concentrations of protein synthesis inhibitors (azetidine-2-carboxylic acid, p-fluorophenylalanine, puromycin), and examined with respect to the effects of the inhibitors on several features of virus-induced cell injury. The virus-specific events in the cells could be dissociated into three groups, based on their sensitivity to the inhibitors: (i) viral ribonucleic acid (RNA) synthesis, bulk viral protein synthesis, and infectious particle production, all of which were prevented by low inhibitor concentrations; (ii) the cytopathic effect (CPE) and stimulation of phosphatidylcholine synthesis, which were sensitive to intermediate concentrations of the inhibitors; and (iii) the virus-induced inhibitions of host RNA and protein synthesis, which were resistart to the inhibitors of protein synthesis except at very high concentrations. It is concluded from this that the virus-induced CPE and stimulation of phosphatidylcholine synthesis are not consequences of the inhibition of cellular RNA or protein synthesis. Analysis of the virus-specific protein and RNA synthesized at several concentrations of azetidine and puromycin suggests that the CPE may be induced by a viral protein precursor. Virus-induced inhibition of host RNA and protein synthesis occurred at azetidine concentrations which blocked the synthesis of over 99.7% of the total viral RNA and over 99% of the viral double-stranded RNA (dsRNA). Calculations show that this would correspond to less than 150 dsRNA molecules per infected cell, resulting in a dsRNA-polysome ratio of less than 1:1,000; this indicates that host protein synthesis cannot be inhibited by an irreversible binding of dsRNA to polysomes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amako K., Dales S. Cytopathology of Mengovirus infection. I. Relationship between cellular disintegration and virulence. Virology. 1967 Jun;32(2):184–200. doi: 10.1016/0042-6822(67)90269-3. [DOI] [PubMed] [Google Scholar]
- Amako K., Dales S. Cytopathology of Mengovirus infection. II. Proliferation of membranous cisternae. Virology. 1967 Jun;32(2):201–215. doi: 10.1016/0042-6822(67)90270-x. [DOI] [PubMed] [Google Scholar]
- BABLANIAN R., EGGERS H. J., TAMM I. STUDIES ON THE MECHANISM OF POLIOVIRUS-INDUCED CELL DAMAGE. I. THE RELATION BETWEEN POLIOVIRUS,-INDUCED METABOLIC AND MORPHOLOGICAL ALTERATIONS IN CULTURED CELLS. Virology. 1965 May;26:100–113. doi: 10.1016/0042-6822(65)90030-9. [DOI] [PubMed] [Google Scholar]
- BABLANIAN R., EGGERS H. J., TAMM I. STUDIES ON THE MECHANISM OF POLIOVIRUS-INDUCED CELL DAMAGE. II. THE RELATION BETWEEN POLIOVIRUS GROWTH AND VIRUS-INDUCED MORPHOLOGICAL CHANGES IN CELLS. Virology. 1965 May;26:114–121. doi: 10.1016/0042-6822(65)90031-0. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Hall L., Stoltzfus C. M., Rueckert R. R. Virus-specific proteins synthesized in encephalomyocarditis virus-infected HeLa cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3083–3087. doi: 10.1073/pnas.68.12.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell-Stewart B., Taylor M. W. Effect of double-stranded viral RNA on mammalian cells in culture. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1326–1330. doi: 10.1073/pnas.68.6.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S., EGGERS H. J., TAMM I., PALADE G. E. ELECTRON MICROSCOPIC STUDY OF THE FORMATION OF POLIOVIRUS. Virology. 1965 Jul;26:379–389. doi: 10.1016/0042-6822(65)90001-2. [DOI] [PubMed] [Google Scholar]
- DALES S., FRANKLIN R. M. A comparison of the changes in fine structure of L cells during single cycles of viral multiplication, following their infection with the viruses of Mengo and encephalomyocarditis. J Cell Biol. 1962 Aug;14:281–302. doi: 10.1083/jcb.14.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfeld E., Hunt T. Double-stranded poliovirus RNA inhibits initiation of protein synthesis by reticulocyte lysates. Proc Natl Acad Sci U S A. 1971 May;68(5):1075–1078. doi: 10.1073/pnas.68.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN R. M., BALTIMORE D. Patterns of macromolecular synthesis in normal and virus-infected mammalian cells. Cold Spring Harb Symp Quant Biol. 1962;27:175–198. doi: 10.1101/sqb.1962.027.001.019. [DOI] [PubMed] [Google Scholar]
- Flanagan J. F. Hydrolytic enzymes in KB cells infected with poliovirus and herpes simplex virus. J Bacteriol. 1966 Feb;91(2):789–797. doi: 10.1128/jb.91.2.789-797.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauntt C. J., Lockart R. Z., Jr Inhibition of Mengo virus by interferon. J Bacteriol. 1966 Jan;91(1):176–182. doi: 10.1128/jb.91.1.176-182.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUSEN P., VERWOERD D. W. STUDIES ON THE MULTIPLICATION OF A MEMBER OF THE COLUMBIA SK GROUP (ME VIRUS) IN L CELLS. III. ALTERATION OF RNA AND PROTEIN SYNTHETIC PATTERNS IN VIRUS-INFECTED CELLS. Virology. 1963 Dec;21:617–627. doi: 10.1016/0042-6822(63)90235-6. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J. Depression of host-controlled RNA synthesis in human cells during poliovirus infection. Proc Natl Acad Sci U S A. 1963 Jan 15;49:23–28. doi: 10.1073/pnas.49.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J. INHIBITION OF HOST CELL MACROMOLECULAR SYNTHESIS BY HIGH MULTIPLICITIES OF POLIOVIRUS UNDER CONDITIONS PREVENTING VIRUS SYNTHESIS. J Mol Biol. 1964 Apr;8:574–581. doi: 10.1016/s0022-2836(64)80012-7. [DOI] [PubMed] [Google Scholar]
- HOMMA M., GRAHAM A. F. SYNTHESIS OF RNA IN L CELLS INFECTED WITH MENGO VIRUS. J Cell Physiol. 1963 Oct;62:179–192. doi: 10.1002/jcp.1030620207. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Levy H., Baron S., Kasel J. A. Mengovirus-induced cytopathic effect in L-cells: protective effect of interferon. J Virol. 1969 Oct;4(4):490–495. doi: 10.1128/jvi.4.4.490-495.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Specific cleavage of viral proteins as steps in the synthesis and maturation of enteroviruses. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1015–1022. doi: 10.1073/pnas.60.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz R., Penman S. Regulation of protein synthesis in HeLa cells. 3. Inhibition during poliovirus infection. J Virol. 1971 Nov;8(5):661–668. doi: 10.1128/jvi.8.5.661-668.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENMAN S. STIMULATION OF THE INCORPORATION OF CHOLINE IN POLIOVIRUS-INFECTED CELLS. Virology. 1965 Jan;25:149–152. doi: 10.1016/0042-6822(65)90263-1. [DOI] [PubMed] [Google Scholar]
- Penman S., Summers D. Effects on host cell metabolism following synchronous infection with poliovirus. Virology. 1965 Dec;27(4):614–620. doi: 10.1016/0042-6822(65)90187-x. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Cleveland P. H., Shea M. A. Effect of mengovirus replication on choline metabolism and membrane formation in novikoff hepatoma cells. J Virol. 1970 Dec;6(6):800–812. doi: 10.1128/jvi.6.6.800-812.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. K., Newman J. F., Rueckert R. R. Synthesis of Maus-Elberfeld viral RNA in ascites tumor cells. J Mol Biol. 1966 Jan;15(1):92–101. doi: 10.1016/s0022-2836(66)80211-5. [DOI] [PubMed] [Google Scholar]
- SALZMAN N. P., LOCKART R. Z., Jr, SEBRING E. D. Alterations in HeLa cell metabolism resulting from poliovirus infection. Virology. 1959 Oct;9:244–259. doi: 10.1016/0042-6822(59)90118-7. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr The decrease in size and synthetic activity of poliovirus polysomes late in the infectious cycle. Virology. 1967 Mar;31(3):427–435. doi: 10.1016/0042-6822(67)90222-x. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Evidence for large precursor proteins in poliovirus synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):966–971. doi: 10.1073/pnas.59.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERWOERD D. W., HAUSEN P. STUDIES ON THE MULTIPLICATION OF A MEMBER OF THE COLUMBIA SK GROUP (ME VIRUS) IN L CELLS. IV. ROLE OF "EARLY PROTEINS" IN VIRUS INDUCED METABOLIC CHANGES. Virology. 1963 Dec;21:628–635. doi: 10.1016/0042-6822(63)90236-8. [DOI] [PubMed] [Google Scholar]
- WOLFF D. A., BUBEL H. C. THE DISPOSITION OF LYSOSOMAL ENZYMES AS RELATED TO SPECIFIC VIRAL CYTOPATHIC EFFECTS. Virology. 1964 Nov;24:502–505. doi: 10.1016/0042-6822(64)90196-5. [DOI] [PubMed] [Google Scholar]
- ZIMMERMAN E. F., HEETER M., DARNELL J. E. RNA synthesis in poliovirus-infected cells. Virology. 1963 Mar;19:400–408. doi: 10.1016/0042-6822(63)90080-1. [DOI] [PubMed] [Google Scholar]


