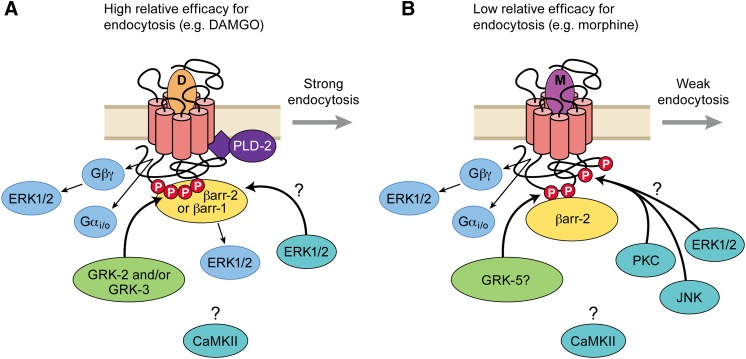
Fig. 5.
Summary of MOR phosphorylation and enzyme interactions leading to desensitization and endocytosis. (A) For agonists with high relative efficacy for endocytosis, G protein dissociation and conformational changes favorable to GRK phosphorylation drive desensitization and endocytosis. GRK2 and GRK3 appear to be the major isoforms involved (see Desensitization and Tolerance Are Both Associated with Reduction of Functional Receptors). Arrestin binding requires GRK phosphorylation, and both β-arrestin2 and β-arrestin1 can interact with MOR to promote endocytosis (see Biased Agonism and μ-Opioid Receptor Regulation; Groer et al., 2011). It is not certain if phosphorylation events, β-arrestin binding, or both produce uncoupling of MOR signaling (desensitization), but the time course appears to follow arrestin binding more closely. Strong internalizing agonists activate phospholipase D2 (Section V.G), but it is not certain whether this is required for endocytosis (Arttamangkul et al., 2012). There is some evidence that ERK1/2-dependent mechanisms may desensitize MOR by both arrestin-independent (Gβγ) and arrestin-dependent mechanisms. Other kinases may also be important, including CAMKII and PKC. GRK phosphorylation is rapidly reversible at the cell surface, but the rate of reversal at other potential phosphorylation sites or their requirements for endocytosis is unknown (see Phosphorylation and μ-Opioid Receptor Regulation). (B) Agonists with relatively low efficacy for endocytosis weakly and slowly phosphorylate GRK substrates on MOR and induce weak association with β-arrestin2 (see Desensitization and Tolerance Are Both Associated with Reduction of Functional Receptors and Biased Agonism and μ-Opioid Receptor Regulation). However, there is good evidence that PKC-dependent mechanisms, possibly via direct phosphorylation of MOR at Thr370 (Doll et al., 2011), contribute to desensitization by agonists such as morphine (Section V.D). PKC also appears to recruit JNK-dependent desensitization mechanisms for agonists such as morphine but not for high endocytosis efficacy agonists (Melief et al., 2010). It is tempting to speculate for agonists with low efficacy for endocytosis that PKC and other kinases can readily interact with the intracellular domain of MOR when it is not occluded either by G proteins or arrestins. Whether these events are rapidly reversible (as is GRK dephosphorylation) is not known.