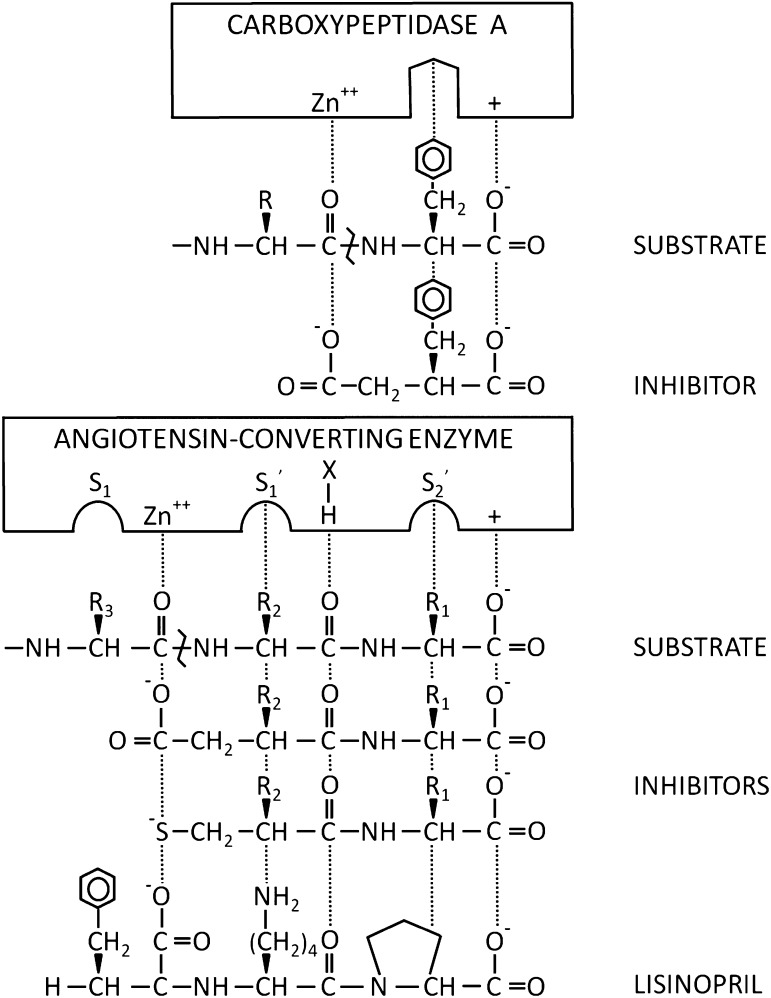
Fig. 5.
Model comparing carboxypeptidase A and ACE. In this schematic, the active sites of carboxypeptidase A and ACE are compared. The pockets in the enzyme represent side chain binding sites within the active sites of the enzymes. In ACE, these pockets are often labeled S1, S1′, and S2′ based on their position relative to the zinc molecule. The + indicate positive charges and X-H represents a potential hydrogen bond. Interactions stabilizing substrates or inhibitors are indicated with dots. The substrate amino bond hydrolyzed by the enzymes is indicated with a bent line. Initial thinking about ACE inhibitors was derived from work showing that benzylsuccinic acid was an inhibitor of carboxypeptidase A (Byers and Wolfenden, 1973). A major advance was the use of a sulfhydryl group to bind to the zinc molecule (Ondetti and Cushmen, 1981). The figure also shows the structure of the ACE inhibitor lisinopril, which has a high affinity for ACE due to interaction with the S1 pocket, in addition to the zinc atom and other structural features of ACE. Adapted with permission from Cushman et al. (1977). Copyright (1978) American Chemical Society.