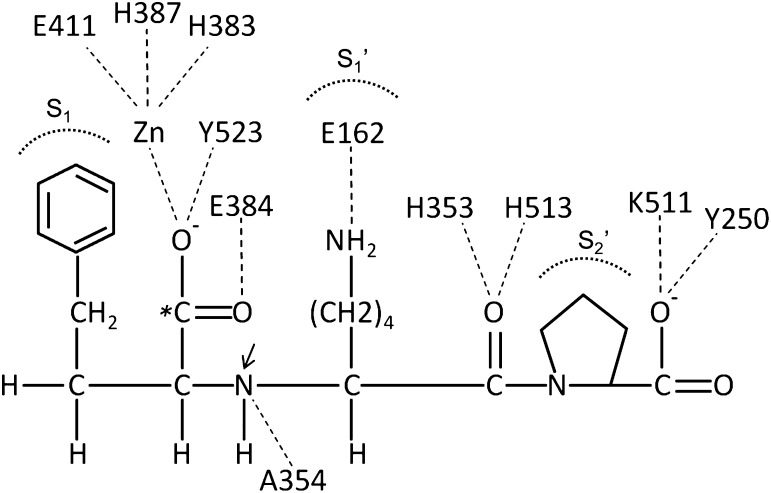
Fig. 6.
Binding of lisinopril to testis ACE. As compared with the schematic in Fig. 5, the crystal structure of human testis ACE–binding lisinopril shows the detailed interactions of individual ACE amino acids with the inhibitor (Natesh et al., 2003). Amino acid numbers are those of testis ACE. Stabilizing interactions are indicated by dashes. The S1-, S1′-, and S2′-binding pockets of ACE are indicated. The carboxyalkyl carboxylate of lisinopril (indicated with an asterisk) substitutes for the amide carbonyl in an ACE substrate. What would be the scissile amide nitrogen in an ACE substrate is indicated by an arrow. Adapted with permission from Macmillan Publishers Ltd: Natesh et al. (2003).