Abstract
Bacterial l-asparaginase has been a universal component of therapies for childhood acute lymphoblastic leukemia since the 1970s. Two principal enzymes derived from Escherichia coli and Erwinia chrysanthemi are the only options clinically approved to date. We recently reported a study of recombinant l-asparaginase (AnsA) from Rhizobium etli and described an increasing type of AnsA family members. Sequence analysis revealed four conserved motifs with notable differences with respect to the conserved regions of amino acid sequences of type I and type II l-asparaginases, particularly in comparison with therapeutic enzymes from E. coli and E. chrysanthemi. These differences suggested a distinct immunological specificity. Here, we report an in silico analysis that revealed immunogenic determinants of AnsA. Also, we used an extensive approach to compare the crystal structures of E. coli and E. chrysantemi asparaginases with a computational model of AnsA and identified immunogenic epitopes. A three-dimensional model of AsnA revealed, as expected based on sequence dissimilarities, completely different folding and different immunogenic epitopes. This approach could be very useful in transcending the problem of immunogenicity in two major ways: by chemical modifications of epitopes to reduce drug immunogenicity, and by site-directed mutagenesis of amino acid residues to diminish immunogenicity without reduction of enzymatic activity.
Keywords: Rhizobium etli, ALL, asparaginase II, immuno-Logo, immunogenic epitopes
Introduction
The last two decades have been characterized by the emergence of a great number of proteins as potential drug candidates to treat diseases in patients with various cancers, or as treatments for heart attacks, strokes, cystic fibrosis, Gaucher’s disease, diabetes, anemia, hemophilia and inflammation. These therapeutic proteins are extracted from plants, microbes or human cells or are engineered in the laboratory for pharmaceutical use.1 The majority of them are manufactured using bacterial systems and nonhuman mammalian cell lines and recombinant techniques,2 and they constitute an important class of therapeutics used to replace patients’ deficiencies in critical blood-borne growth factors and to strengthen the immune system to fight cancer and infectious diseases.3 Twenty-nine years have passed since approval of recombinant human insulin by the FDA, and a remarkable expansion has been seen in the number of therapeutic applications of proteins.4,5 Today we are witnessing a continuous rise in the number of approved protein therapeutics,6 and there is little doubt that biopharmaceuticals have the potential to become the medicines of the future. To date, more than 130 proteins (over 95 of which are recombinant) are currently approved for use by the FDA, and many more are in development.7,8 Recombinant DNA technology not only allows therapeutic proteins to be produced on a large scale but also, by using the same methodology, protein molecules can be engineered and improved.9 The genetic modifications introduced into a protein have many advantages over chemical modifications, since engineered entities must be considered biocompatible and biodegradable. In conjunction, changes introduced into a molecule avoid rare errors in gene transcription or translation, and protein preparations do not contain residual amounts of harsh chemicals used in the purification process.10
From a therapeutic perspective, proteins offer the distinct advantage of specific mechanisms of action and high potency. Despite these advantages, biotech products must overcome the hurdles posed by their high molecular weights, short half-lives, instability and immunogenicity. Several strategies have been evaluated in an effort to improve the current limitations of therapeutic peptides and proteins in the creation of so-called “second-generation” protein therapeutics. Most efforts are centered on two approaches: either a change in the agent itself (e.g., mutations in protein structure or covalent attachment of moieties)11 or by a change in formulation.12 In contrast to modifying the protein structure, covalent chemical attachment of compounds, such as polyethylene glycol (PEG) or polysialic acid (PSA), to a therapeutic protein represents a relatively new approach. Drug formulation systems, such as liposomes, polymeric microspheres and polymeric nanoparticles, are another means to help overcome the current limitations of protein therapeutics.13 A third strategy to minimize immunogenicity of therapeutic proteins is related to identification of immunogenic epitopes on the protein by using in silico prediction tools and by bioengineering less immunogenic proteins.1,14
Immunogenicity of Therapeutic Proteins
Therapeutic proteins can be classified by their function or application into four main groups: (1) proteins with enzymatic or regulatory activity, (2) proteins with special targeting activity, (3) protein vaccines and (4) Proteins used for disease diagnosis.15
Protein therapeutics have several advantages over small-molecule drugs: proteins are highly specific and carry on a complex set of functions that cannot be mimicked by simple chemical compounds; all of them are highly specific with less potential to interfere with normal biological processes; often they are well tolerated and are less likely to elicit an immune response; they can provide effective replacement treatment options without the need for gene therapy; the approval times for therapeutic proteins may be faster than those for small-molecule drugs; and finally, proteins are unique in form and are able to obtain far-reaching patent protections.7,16
The attractiveness of therapeutic proteins in fact is related to the high specificity by which they execute diverse functions.17 The introduction of recombinant human proteins, such as recombinant human erythropoietin, insulin proteins, growth hormones and cytokines, has revolutionized the treatment of many diseases.18-20 It is currently estimated that there are 25,000 to 40,000 different genes in the human genome, which suggests that many more therapeutic proteins will soon become available. However, administration of therapeutic proteins in multiple doses over extended periods has been shown to induce immune responses. These immune responses can be as minor as local irritation or as serious as cardiovascular collapse.21,22 Most biotechnologically derived proteins induce an unwanted immune response that is triggered by more than a single factor. The immunological response is complex and, in addition to antibody formation, other events, such as T-cell activation or innate immune response activation, can contribute to potential adverse responses.23 The consequences of an immune reaction range from the transient appearance of antibodies without any clinical significance to severe life-threatening conditions.24 Additional factors that may influence the immunogenicity of therapeutic proteins are attributable to the patient, the disease, or components of products. Factors in patients include underlying disease, genetic background, immune status (including immunomodulating therapy) and dosing schedule. Components and production steps also influence the likelihood of an immune response, based on the manufacturing process, the formulation and stability characteristics. In the case of a recombinant protein, many factors can contribute to alterations in a protein’s ability to elicit an antibody response, including glycosylation, contaminants, temperature changes and storage media.25
l-Asparaginase: A Potent Antitumor Enzyme
A major potential application of therapeutic enzymes is in cancer treatment. These enzymes fall into two categories of therapeutic proteins: agents that degrade the small molecules for which neoplastic tissues have a requirement, and agents that degrade macromolecules. Important features that distinguish enzyme drugs from other types of drugs include enzymes often binding and acting on their targets with great affinity and specificity and enzymes being catalytic and able to convert multiple molecules to the desired product(s).26
Acute lymphoblastic leukemia (ALL) involves the malignant transformation of a clone of cells from the bone marrow, where early lymphoid precursors proliferate and can replace normal cells. For several years, childhood ALL treatment included the use of asparaginases, since lymphoma cells cannot synthesize l-asparagine (l-Asn) and thus depend on external uptake of this amino acid for growth.27,28 Asparaginase has proved promising for the treatment of ALL, considering that tumor cells are deficient in aspartate-ammonia ligase activity, which restricts their ability to synthesize the normally nonessential amino acid l-asparagine. The action of asparaginase does not affect the functioning of normal cells, which are able to synthesize enough for their own requirements, but it reduces the free exogenous l-Asn concentration and so it induces a state of fatal starvation in susceptible tumor cells. These bacterial-type asparaginases have shown a beneficial pharmacological effect in the treatment of ALL by depleting blood l-Asn pools.29-33 Nowadays, l-asparaginases from Escherichia coli and Erwinia chrysanthemi are used in the treatment of ALL.34 Their therapeutic efficacy is well established. However, in some patients their beneficial effect rarely occurs without some evidence of toxicity, which in part is due to the glutaminase activity of these enzymes35,36 and also, primarily, because both native and modified PEG-asparaginase versions of the enzyme are immunogenic, leading to adverse immune responses to both versions.37 With these factors in mind, l-asparaginases with high specificity for l-Asn and negligible activity against l-glutamine are indicated for anticancer therapy.38-40 Several asparaginases have been investigated in order to characterize enzymes with less toxic side effects.41-44
Recently, we reported that l-asparaginase from Rhizobium etli (AnsA) hydrolyzes l-Asn at similar levels as the l-asparaginase from E. chrysanthemi.45 With regard to substrate specificity, enzymatic activities were not detected for aspartate, d-asparagine or glutamine as substrates. The activity of AnsA was specific for l-Asn. We also confirmed that AnsA does not show glutaminase activity. In addition, we described 350 proteins of the rhizobial-type asparaginases grouped in this subfamily, wherein the majority are derived from microorganisms that inhabit soil or marine environments. Sequence analysis revealed four conserved motifs [RSx(2)KPxQA; ALxCASH; NCSGKHxGxL; and AMx(3)Px(2)VAGxGrx(2)TxLM], with notable differences with respect to the conserved regions of amino acid sequences of type I and type II l-asparaginases, particularly on comparison with therapeutic enzymes of E. coli and E. chrysanthemi. These differences suggest a distinct immunological specificity. In summary, considering the biochemical properties of l-asparaginase of R. etli, e.g., the glutaminase-free asparaginase activity, and its different amino acid sequence compared with E. coli and E. chrysantemi asparaginases, led us to propose it as a potential therapeutic enzyme for ALL treatment.46
In Silico Immunogenicity Profile of Rhizobial l-asparaginase (AnsA)
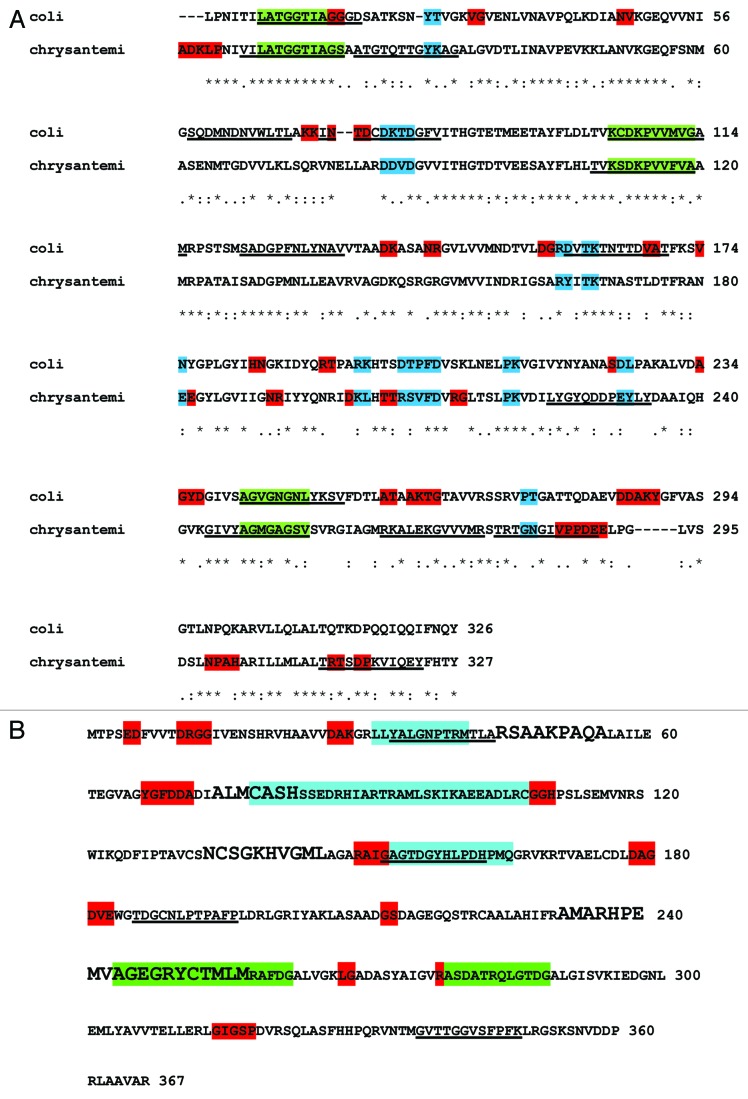
In order to determine the immunogenic determinants of asparaginases, we used an in silico approach to compare amino acid sequences and immunogenic profiles of the three enzymes. The first question we asked was if E. coli and E. chrysantemi asparaginases are phylogenetically and immunogenically closely related. To this end, sequence comparison among asparaginases was performed by BLAST analysis (http://blast.ncbi.nlm.nih.gov). E. coli and E. chrysantemi asparaginases were closely related (47% identity; Figure 1A), whereas AnsA showed completely different sequence (6% identity) from the other two asparaginases. A subsequent analysis of the amino acid sequences of the three enzymes was performed to investigate antigenic determinants (epitopes) by using the servers BCPREDS (B-cell epitope prediction server; http://ailab.cs.iastate.edu/bcpreds/)47,48 and Epitopia (http://epitopia.tau.ac.il/). The antigenic determinants are shown in Table 1.
Figure 1. (A) Immunogenic profiles of the E. coli and E. chrysantemi asparaginases. Highly immunogenic residues identified by using Epitopia are shown (shaded residues). Red, no shared epitopes; blue, shared epitopes. Immunogenic epitopes identified by using BCPREDS are underlined. Shared epitopes predicted by BCPREDS are shown in green. (B) Immunogenic profiles of AnsA. Residues in red had the highest Epitopia scores. BCPREDS epitopes are indicated by underlining. Four conserved motifs from rhizobial-type asparaginases are shown in boldface letters. Cyan, score = 0.7 to 0.79; green, score ≥ 8. Shaded residues show antigenic motifs from rhizobial-type asparaginases, identified using Immuno-Logo.
Table 1. BCPreds prediction of B-cell epitopes from asparaginases.
| Epitope sequences of L-asparaginases | |||
|---|---|---|---|
| Amino acid position |
BCPred epitope sequence |
BCPred score |
|
| Rhizobium etli L-asparaginase | |||
| 186 |
TDGCNLPTPAFP |
0.988 |
|
| 150 |
GAGTDGYHLPDH |
0.970 |
|
| 338 |
GVTTGGVSFPFK |
0.929 |
|
| 35 |
YALGNPTRMTLA |
0.813 |
|
| Escherichia coli L-asparaginase | |||
|---|---|---|---|
| 7 |
LATGGTIAGGGD |
1 |
|
| 104 |
KCDKPVVMVGAM |
0.99 |
|
| 242 |
AGVGNGNLYKSV |
0.984 |
|
| 73 |
INTDCDKTDGFV |
0.945 |
|
| 159 |
DVTKTNTTDVAT |
0.898 |
|
| 58 |
SQDMNDNVWLTL |
0.891 |
|
| 122 |
SADGPFNLYNAV |
0.81 |
|
| Erwinia chrysantemi L-asparaginase | |||
|---|---|---|---|
| 312 |
TRTSDPKVIQEY |
0.991 |
|
| 108 |
TVKSDKPVVFVA |
0.983 |
|
| 277 |
TRTGNGIVPPDE |
0.972 |
|
| 21 |
ATGTQTTGYKAG |
0.958 |
|
| 244 |
GIVYAGMGAGSV |
0.934 |
|
| 264 |
RKALEKGVVVMR |
0.898 |
|
| 223 |
LYGYQDDPEYLY |
0.883 |
|
| 8 | VILATGGTIAGS | 0.802 | |
Epitope length: 12 amino acids, and specificity at 75%. Only BCPRED score values ≥ 0.8 were considered.
The immunogenic epitopes with higher values were mapped in the E. coli and E. chrysantemi asparaginase protein sequences (Fig. 1A). Shaded residues represent the most highly immunogenic determinants predicted by Epitopia analysis, whereas underlined epitopes show BCPREDS predictions. As expected, E. coli and E. chrysantemi asparaginases shared immunogenic residues (Fig. 1A, blue and green), and at least five of them were predicted by two servers (Fig. 1, green). In order to evaluate the conservation of immunogenic regions in AsnA and its relatives, a BLAST search was run using AsnA of R. etli as seed, with an E-value ≤ 10−5, against the NR database. A total of 372 protein sequences were retrieved from this sequence comparison. These sequences were filtered at 90% identity to exclude overrepresentation biases by using the CD-hit program,49 leaving a set of 284 proteins. These sequences were then aligned by using the CLUSTAL program50 with default parameters, and their corresponding immunogenic regions were located by Epitopia analysis (Immuno-Logo; Fig. S1). From this comparison we found three motifs with high immunogenicity values (score, > 0.8) (Fig. 1B, cyan) and two with a score between 0.7 and 0.8 (Fig. 1B, green). We also found that two rhizobial-type family conserved motifs previously identified overlap with the immunogenic regions, suggesting that AnsA immunogenic properties may be a general property for AsnA homologs of R. etli (Fig. 1B).
Structural Comparison of E. coli, E. chrysantemi, and R. etli Asparaginases
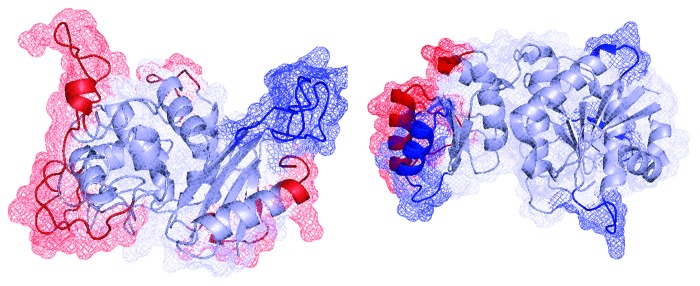
The crystal structures of E. coli asparaginase (PDB entry 1JJA) and E. chrysantemi (PDB entry 1HG1) were analyzed. These structures were compared using the structural alignment Topmatch server (http://topmatch.services.came.sbg.ac.at). Additionally, a computational model of the three-dimensional structure of AsnA was built by using the CPHmodels 3.2 server (http://www.cbs.dtu.dk/services/CPHmodels) with the structure of OXA10, a class d β-lactamase from Pseudomonas aeruginosa (PDB entry 1E3U.D), as a template. In order to refine the model, it was minimized using the Gromacs server (http://lorentz.immstr.pasteur.fr/gromacs). Finally, the RAMPAGE program (http://mordred.bioc.cam.ac.uk/~rapper/rampage.php) was used to validate the stereochemical quality of the resulting three-dimensional model. After analyzing the Ramachandran plot, 88.5% of the residues were in favored and allowed regions, and only 7.6% were in disallowed regions. All the bond distances, angles, and dihedrals fulfilled the normal limits for polypeptide chains. The model includes 314 of the 371 residues of AsnA. The three-dimensional model of R. etli asparaginase was then compared with crystal structures of the E. coli and E. chrysantemy asparaginases (Fig. 2). Structurally, AsnA has a strong similarity to proteins belonging to the β-lactamase/transpeptidase-like fold. In contrast, asparaginases from E. coli and E. chrysantemi belong to the glutaminase/asparaginase fold, suggesting different evolutionary origins or a large divergence in terms of sequence and structure. In order to corroborate this finding, we performed a structural superposition of AsnA of the three organisms, with similar results, reinforcing the notion that they share different structural determinants, i.e., the superposition RMSD values in all α carbons showed high structural similarities between E. coli and E. chrysantemi asparaginases (RMSD = 1.68 Å), whereas both proteins were dissimilar to AsnA (RMSD = 21.86 Å). High RMSD values indicate a lack of structural correspondence. In order to identify continuous epitopes in the proteins, we used the ElliPro server. In brief, this method identifies potential epitopes protruding from the protein’s globular surface and, together with a residue clustering algorithm, allows the prediction of antibody epitopes for a given protein.51 The comparison of crystal structures of E. coli and E. chrysantemi asparaginases regarding the three-dimensional model of AsnA revealed, as expected based on sequence dissimilarities, a completely different folding and different immunogenic epitopes.
Figure 2. Computational model of the R. etli AsnA (left) and the crystal structure of the E. chrysantemi asparaginase (right). Immunogenic regions with a score of ≥ 0.8 are shown in blue. In red are immunogenic regions with scores between 0.7 and 0.799. Both proteins exhibited a different fold and different immunogenic determinants.
Conclusions
The computational prediction of therapeutic protein epitopes is of important theoretical and practical value, as experimental identification of such epitopes is costly and time-consuming. This approach could be useful in reducing the problem of immunogenicity with the use of therapeutic proteins. First, we can identify those immunogenic residues included in conserved motifs that are presumably involved in enzymatic activity or protein stability. Identification of such residues will allow us to change by site-directed mutagenesis the nonconserved residues to reduce epitope immunogenicity without loss of enzymatic activity or stability. Second, the conserved residues with high immunogenicity could be subjected to covalent chemical attachment of compounds, such as PEG or PSA, to reduce immunogenicity. On the other hand, evaluation of conserved immunogenic regions is a strong approach to evaluate whether any given therapeutic protein is a suitable candidate to be included in a disease treatment or if its immunogenic profile warns us about its use as an alternative for clinical treatment.
Supplementary Material
Acknowledgments
E.P.-R. gratefully acknowledge the support provided by CONACYT grant number 155116. A.H.-S. was partially supported by DGAPA-IACOD grant I1202711.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental materials can be found here: www.landesbioscience.com/journals/bioe/article/21710/
Footnotes
Previously published online: www.landesbioscience.com/journals/bioe/article/21710
References
- 1.Adair F, Ozanne D. The immunogenicity of therapeutic proteins. Biopharm International. 2002;15:30–6. [Google Scholar]
- 2.Matasci M, Hacker DL, Baldi L, Wurm FM. Recombinant therapeutic protein production in cultivated mammalian cells: current status and future prospects. Drug Discov Today. 2008;5:e37–42. doi: 10.1016/j.ddtec.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Nayak KA. Advances in therapeutic protein production and delivery. Int J Pharmacy and Pharma Sci. 2010;2:1–5. [Google Scholar]
- 4.Johnson IS. Human insulin from recombinant DNA technology. Science. 1983;219:632–7. doi: 10.1126/science.6337396. [DOI] [PubMed] [Google Scholar]
- 5.Purcell RT, Lockey RF. Immunologic responses to therapeutic biologic agents. J Investig Allergol Clin Immunol. 2008;18:335–42. [PubMed] [Google Scholar]
- 6.Walsh G. Biopharmaceutical benchmarks 2006. Nat Biotechnol. 2006;24:769–76. doi: 10.1038/nbt0706-769. [DOI] [PubMed] [Google Scholar]
- 7.Leader B, Baca QJ, Golan DE. Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov. 2008;7:21–39. doi: 10.1038/nrd2399. [DOI] [PubMed] [Google Scholar]
- 8.Baker MP, Reynolds HM, Lumicisi B, Bryson CJ. Immunogenicity of protein therapeutics: The key causes, consequences and challenges. Self Nonself. 2010;1:314–22. doi: 10.4161/self.1.4.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomlinson IM. Next-generation protein drugs. Nat Biotechnol. 2004;22:521–2. doi: 10.1038/nbt0504-521. [DOI] [PubMed] [Google Scholar]
- 10.Kamionka M. Engineering of therapeutic proteins production in Escherichia coli. Curr Pharm Biotechnol. 2011;12:268–74. doi: 10.2174/138920111794295693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenkins N. Modifications of therapeutic proteins: challenges and prospects. Cytotechnology. 2007;53:121–5. doi: 10.1007/s10616-007-9075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soderquist RG, Milligan ED, Sloane EM, Harrison JA, Douvas KK, Potter JM, et al. PEGylation of brain-derived neurotrophic factor for preserved biological activity and enhanced spinal cord distribution. J Biomed Mater Res A. 2009;91:719–29. doi: 10.1002/jbm.a.32254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banakar UV. Advances and opportunities in delivery of therapeutic proteins and peptides. J Biomater Appl. 1997;11:377–429. doi: 10.1177/088532829701100402. [DOI] [PubMed] [Google Scholar]
- 14.De Groot AS, Martin W. Reducing risk, improving outcomes: bioengineering less immunogenic protein therapeutics. Clin Immunol. 2009;131:189–201. doi: 10.1016/j.clim.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Sutradhar KB, Khatun S, Al Mamun A, Begum M. Distribution and elimination of protein therapeutics: a review. S J Pharm Sci. 2011;4:1–12. [Google Scholar]
- 16.Carter PJ. Introduction to current and future protein therapeutics: a protein engineering perspective. Exp Cell Res. 2011;317:1261–9. doi: 10.1016/j.yexcr.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Wright CM, Wright RC, Eshleman JR, Ostermeier M. A protein therapeutic modality founded on molecular regulation. Proc Natl Acad Sci U S A. 2011;108:16206–11. doi: 10.1073/pnas.1102803108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oberg K, Alm G, Magnusson A, Lundqvist G, Theodorsson E, Wide L, et al. Treatment of malignant carcinoid tumors with recombinant interferon alfa-2b: development of neutralizing interferon antibodies and possible loss of antitumor activity. J Natl Cancer Inst. 1989;81:531–5. doi: 10.1093/jnci/81.7.531. [DOI] [PubMed] [Google Scholar]
- 19.Gelfand EW. Antibody-directed therapy: past, present, and future. J Allergy Clin Immunol. 2001;108(Suppl):S111–6. doi: 10.1067/mai.2001.117824. [DOI] [PubMed] [Google Scholar]
- 20.Olsen E, Duvic M, Frankel A, Kim Y, Martin A, Vonderheid E, et al. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J Clin Oncol. 2001;19:376–88. doi: 10.1200/JCO.2001.19.2.376. [DOI] [PubMed] [Google Scholar]
- 21.Gruchalla R. Understanding drug allergies. J Allergy Clin Immunol. 2000;105:S637–44. doi: 10.1067/mai.2000.106156. [DOI] [PubMed] [Google Scholar]
- 22.Schellekens H. Factors influencing the immunogenicity of therapeutic proteins. Nephrol Dial Transplant. 2005;20(Suppl 6):vi3–9. doi: 10.1093/ndt/gfh1092. [DOI] [PubMed] [Google Scholar]
- 23.Cantor JR, Yoo TH, Dixit A, Iverson BL, Forsthuber TG, Georgiou G. Therapeutic enzyme deimmunization by combinatorial T-cell epitope removal using neutral drift. Proc Natl Acad Sci U S A. 2011;108:1272–7. doi: 10.1073/pnas.1014739108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chirino AJ, Ary ML, Marshall SA. Minimizing the immunogenicity of protein therapeutics. Drug Discov Today. 2004;9:82–90. doi: 10.1016/S1359-6446(03)02953-2. [DOI] [PubMed] [Google Scholar]
- 25.Sharma B. Immunogenicity of therapeutic proteins. Part 2: impact of container closures. Biotechnol Adv. 2007;25:318–24. doi: 10.1016/j.biotechadv.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Sabu A. Sources, properties and applications of microbial therapeutic enzymes. Int J Biotechnol. 2003;2:334–41. [Google Scholar]
- 27.Cooney DA, Handschumacher RE. L-asparaginase and L-asparagine metabolism. Annu Rev Pharmacol. 1970;10:421–40. doi: 10.1146/annurev.pa.10.040170.002225. [DOI] [PubMed] [Google Scholar]
- 28.Cantor JR, Panayiotou V, Agnello G, Georgiou G, Stone EM. Engineering reduced-immunogenicity enzymes for amino acid depletion therapy in cancer. Methods Enzymol. 2012;502:291–319. doi: 10.1016/B978-0-12-416039-2.00015-X. [DOI] [PubMed] [Google Scholar]
- 29.Boyse EA, Old LJ, Campbell HA, Mashburn LT. Suppression of murine leukemias by L-asparaginase. Incidence of sensitivity among leukemias of various types: comparative inhibitory activities of guinea pig serum L-asparaginase and Escherichia coli L-asparaginase. J Exp Med. 1967;125:17–31. doi: 10.1084/jem.125.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell HA, Mashburn LT, Boyse EA, Old LJ. Two L-asparaginases from Escherichia coli B. Their separation, purification, and antitumor activity. Biochemistry. 1967;6:721–30. doi: 10.1021/bi00855a011. [DOI] [PubMed] [Google Scholar]
- 31.Gallagher MP, Marshall RD, Wilson R. Asparaginase as a drug for treatment of acute lymphoblastic leukaemia. Essays Biochem. 1989;24:1–40. [PubMed] [Google Scholar]
- 32.Mashburn LT, Wriston JC., Jr. Tumor Inhibitory effect of L-asparaginase from Escherichia coli. Arch Biochem Biophys. 1964;105:450–2. doi: 10.1016/0003-9861(64)90032-3. [DOI] [PubMed] [Google Scholar]
- 33.Roberts J, Prager MD, Bachynsky N. The antitumor activity of Escherichia coli L-asparaginase. Cancer Res. 1966;26:2213–7. [PubMed] [Google Scholar]
- 34.Dinndorf PA, Gootenberg J, Cohen MH, Keegan P, Pazdur R. FDA drug approval summary: pegaspargase (oncaspar) for the first-line treatment of children with acute lymphoblastic leukemia (ALL) Oncologist. 2007;12:991–8. doi: 10.1634/theoncologist.12-8-991. [DOI] [PubMed] [Google Scholar]
- 35.Ashworth LA, MacLennan AP. Comparison of the L-asparaginases from Escherichia coli and Erwinia carotovora as immunosuppressants. Cancer Res. 1974;34:1353–9. [PubMed] [Google Scholar]
- 36.Moola ZB, Scawen MD, Atkinson T, Nicholls DJ. Erwinia chrysanthemi L-asparaginase: epitope mapping and production of antigenically modified enzymes. Biochem J. 1994;302:921–7. doi: 10.1042/bj3020921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeidan A, Wang ES, Wetzler M. Pegasparaginase: where do we stand? Expert Opin Biol Ther. 2009;9:111–9. doi: 10.1517/14712590802586058. [DOI] [PubMed] [Google Scholar]
- 38.Distasio JA, Niederman RA, Kafkewitz D. Antilymphoma activity of a glutaminase-free L-asparaginase of microbial origin. Proc Soc Exp Biol Med. 1977;155:528–31. doi: 10.3181/00379727-155-39844. [DOI] [PubMed] [Google Scholar]
- 39.Hawkins DS, Park JR, Thomson BG, Felgenhauer JL, Holcenberg JS, Panosyan EH, et al. Asparaginase pharmacokinetics after intensive polyethylene glycol-conjugated L-asparaginase therapy for children with relapsed acute lymphoblastic leukemia. Clin Cancer Res. 2004;10:5335–41. doi: 10.1158/1078-0432.CCR-04-0222. [DOI] [PubMed] [Google Scholar]
- 40.Kumar S, Venkata Dasu V, Pakshirajan K. Purification and characterization of glutaminase-free L-asparaginase from Pectobacterium carotovorum MTCC 1428. Bioresour Technol. 2011;102:2077–82. doi: 10.1016/j.biortech.2010.07.114. [DOI] [PubMed] [Google Scholar]
- 41.Abdel-Fattah YR, Olama ZA. L-Asparaginase production by Pseudomonas aeruginosa in solid-state culture: Evaluation and optimization of culture conditions using factorial designs. Process Biochem. 2002;38:115–22. doi: 10.1016/S0032-9592(02)00067-5. [DOI] [Google Scholar]
- 42.Elzainy TA, Ali TH. Detection of the antitumor glutaminase-asparaginase in the filamentous fungi. J Appl Sci. 2006;6:1389–95. doi: 10.3923/jas.2006.1389.1395. [DOI] [Google Scholar]
- 43.Fisher SH, Wray LV., Jr. Bacillus subtilis 168 contains two differentially regulated genes encoding L-asparaginase. J Bacteriol. 2002;184:2148–54. doi: 10.1128/JB.184.8.2148-2154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mukherjee J, Majumdar S, Scheper T. Studies on nutritional and oxygen requirements for production of L-asparaginase by Enterobacter aerogenes. Appl Microbiol Biotechnol. 2000;53:180–4. doi: 10.1007/s002530050006. [DOI] [PubMed] [Google Scholar]
- 45.Kotzia GA, Labrou NE. L-Asparaginase from Erwinia Chrysanthemi 3937: cloning, expression and characterization. J Biotechnol. 2007;127:657–69. doi: 10.1016/j.jbiotec.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 46.Moreno-Enriquez A, Evangelista-Martinez Z, González-Mondragon EG, Calderón-Flores A, Arreguín R, Perez-Rueda E, et al. Biochemical characterization of recombinant L-asparaginase (AnsA) from Rhizobium etli, a member of an increasing rhizobial-type family of L-asparaginases. J Microbiol Biotechnol. 2012;22:292–300. doi: 10.4014/jmb.1107.07047. [DOI] [PubMed] [Google Scholar]
- 47.Saha S, Raghava GPS. BcePred:Prediction of Continuous B-Cell Epitopes in Antigenic Sequences Using Physico-chemical Properties. In Nicosia G, Cutello V, Bentley PJ, Timis J, eds. ICARIS. LNCS 3239. Springer, 2004: 197-204. [Google Scholar]
- 48.El-Manzalawy Y, Dobbs D, Honavar V. Predicting linear B-cell epitopes using string kernels. J Mol Recognit. 2008;21:243–55. doi: 10.1002/jmr.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li W, Jaroszewski L, Godzik A. Clustering of highly homologous sequences to reduce the size of large protein database. Bioinformatics. 2001;17:282–3. doi: 10.1093/bioinformatics/17.3.282. [DOI] [PubMed] [Google Scholar]
- 50.Thompson JD, Gibson TJ, Higgins DG. Multiple sequence alignment using ClustalW and 4 ClustalX. Curr Prot Bioinformatics 2002; Chapter 2. Unit 2.3. [DOI] [PubMed] [Google Scholar]
- 51.Ponomarenko J, Bui HH, Li W, Fusseder N, Bourne PE, Sette A, et al. ElliPro: a new structure-based tool for the prediction of antibody epitopes. BMC Bioinformatics. 2008;9:514. doi: 10.1186/1471-2105-9-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.