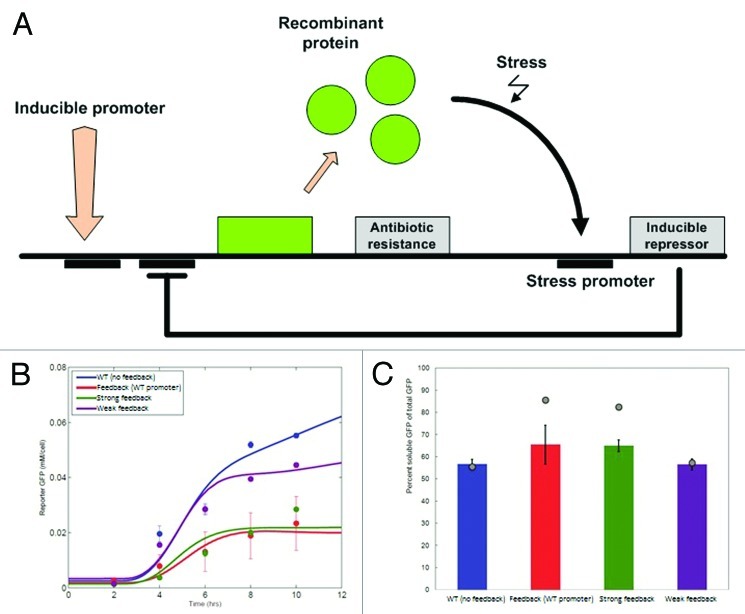
Figure 2. (A) Overview of a self-regulatory system for recombinant protein production. An inducible promoter is used to turn-on recombinant protein production. When the cell is stressed, a strong repressor that is under the control of a stress reporter will be expressed, which will decrease or shut down the protein production. In our proof-of-principle experiments, we placed the recombinant protein (GFP) under a T7 promoter that would be repressed by LacI. The stress-induced promoter for heat shock proteins IbpA and IbpB was used to drive the TetR expression, which in turn adaptively regulated the production of GPF by binding to its promoter. (B) Simulation (solid lines) and experimental (dots) results for the non-feedback system (blue line) and three feedback variations based on different stress promoter variants (engineered through site-directed mutagenesis). Interestingly, the wild-type stress promoter exhibited large variation in its expression, while the engineered promoters are fairly invariant in their effect. (C) Soluble vs. insoluble fraction of the recombinant protein (GPF). Both experimental results (bars) and computational predictions (gray points) are shown for the three representative variations depicted in (B). The difference between the experimentally measured and computationally derived values likely stem from the fact that in the model we don’t account for the effect of TetR proteins in the depletion of the cellular resources and folding machinery.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.